Sucking lice (Phthiraptera: Anoplura) are permanent, obligate, and hematophagous ectoparasites of mammals. Throughout their evolutionary history, they have established associations and co-evolved with mammals, being present in most Mammalian genera. Seal lice is the common name given to a group of sucking lice belonging to the family Echinophthiriidae (Phthiraptera: Anoplura). This group characterises by infesting hosts with an aquatic lifestyle, i.e. pinnipeds (seals, sea lions, and walrus) and the North American river otter.
- adaptation
- Anoplura
- Echinophthiriidae
- extreme environments
1. Introduction
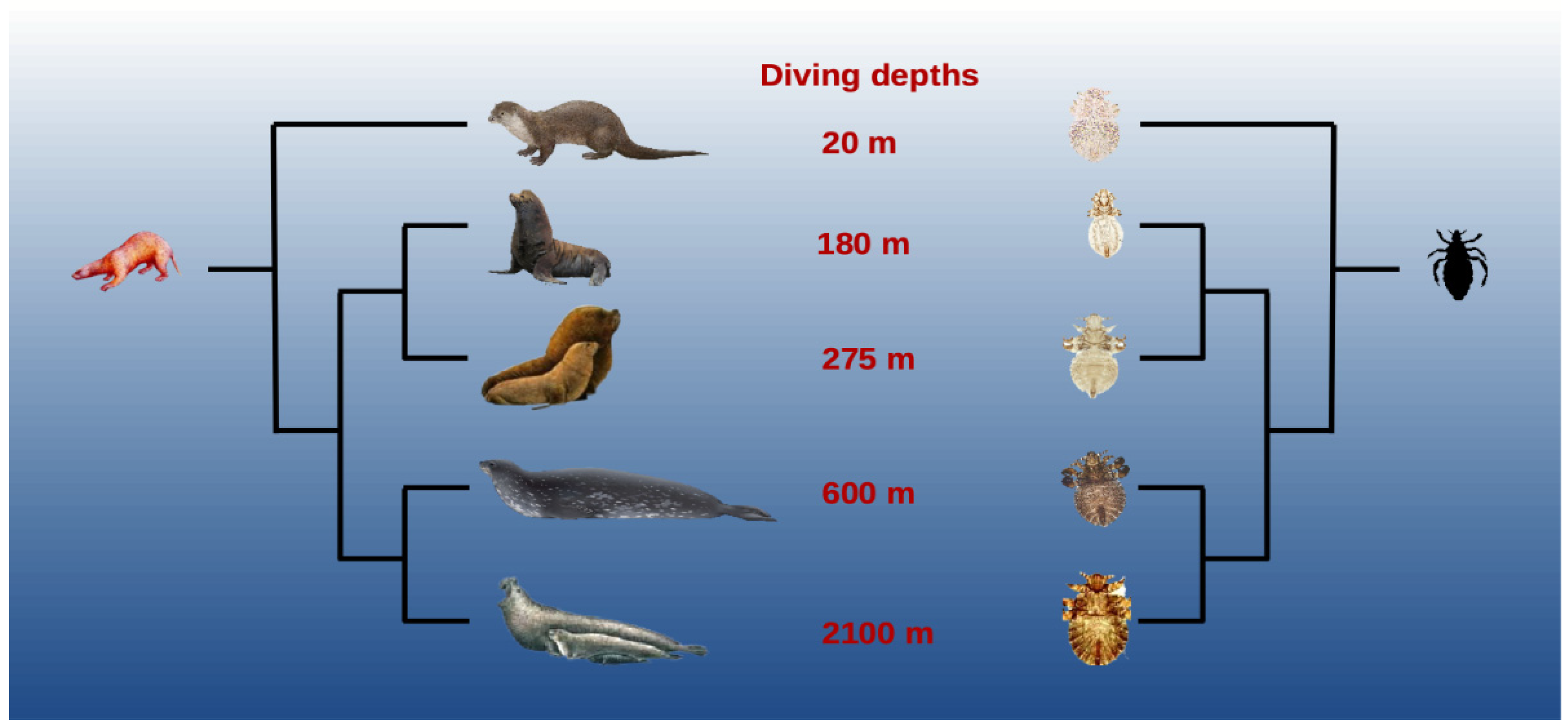
It is important to underline that, equally to their hosts, some species are at risk of extinction, as L. piriformis from monk seals. Further research is needed in order to elucidate the taxonomic status of lice species infesting several species of hosts. In this sense, recent phylogenomic analysis suggests that Antarctophthirus microchir from the five species of existent sea lions are in fact several different species (Leonardi et al., 2019).
2. How Did Seal Lice Turn into the Only Truly Marine Insects?
2.1. Evolution
| Louse Genus | Species | Host |
|---|---|---|
| Antarctophthirus | A. callorhini | Northern fur seal |
| A. carlinii | Weddell seal | |
| A. lobodontis | Crabeater seal | |
| A. mawsoni | Ross seal | |
| A. microchir | Steller, Californian, South American, Australian, and New Zealand sea lion | |
| A. ogmorhini | Leopard seal | |
| A. trichechi | Walrus | |
| Latagophthirus | La. rauschi | North American river otter |
| Lepidophthirus | Le. macrorhini | Elephant seals |
| Le. piriformis | Monk seals | |
| Echinophthirius | E. horridus | Northern true seals |
| Proechinophthirus | P. fluctus | Northern fur seal |
| P. zumpti | Southern fur seals |
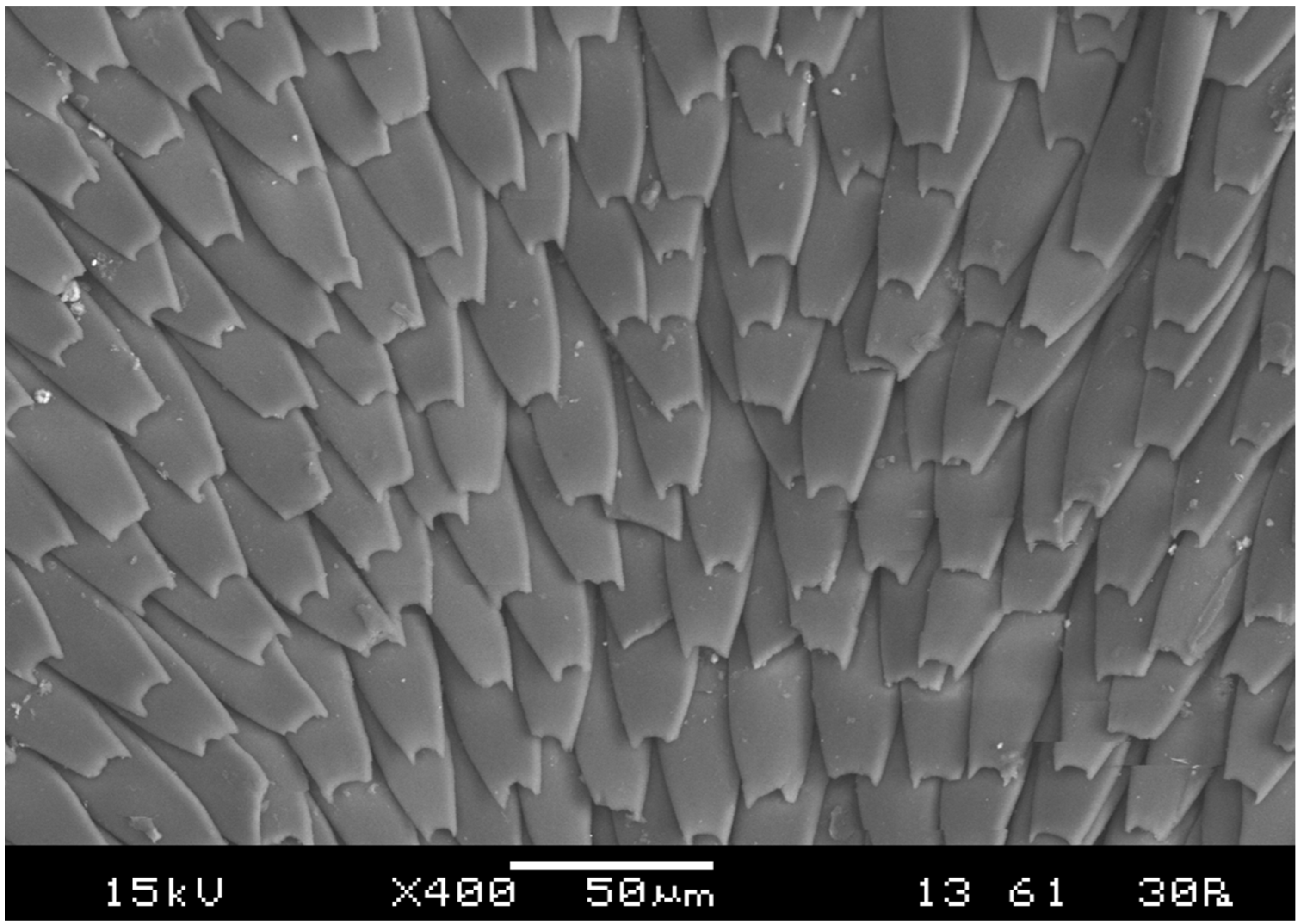
2.2. Morphological Adaptations
2.3. Reproductive Synchronization with Hosts
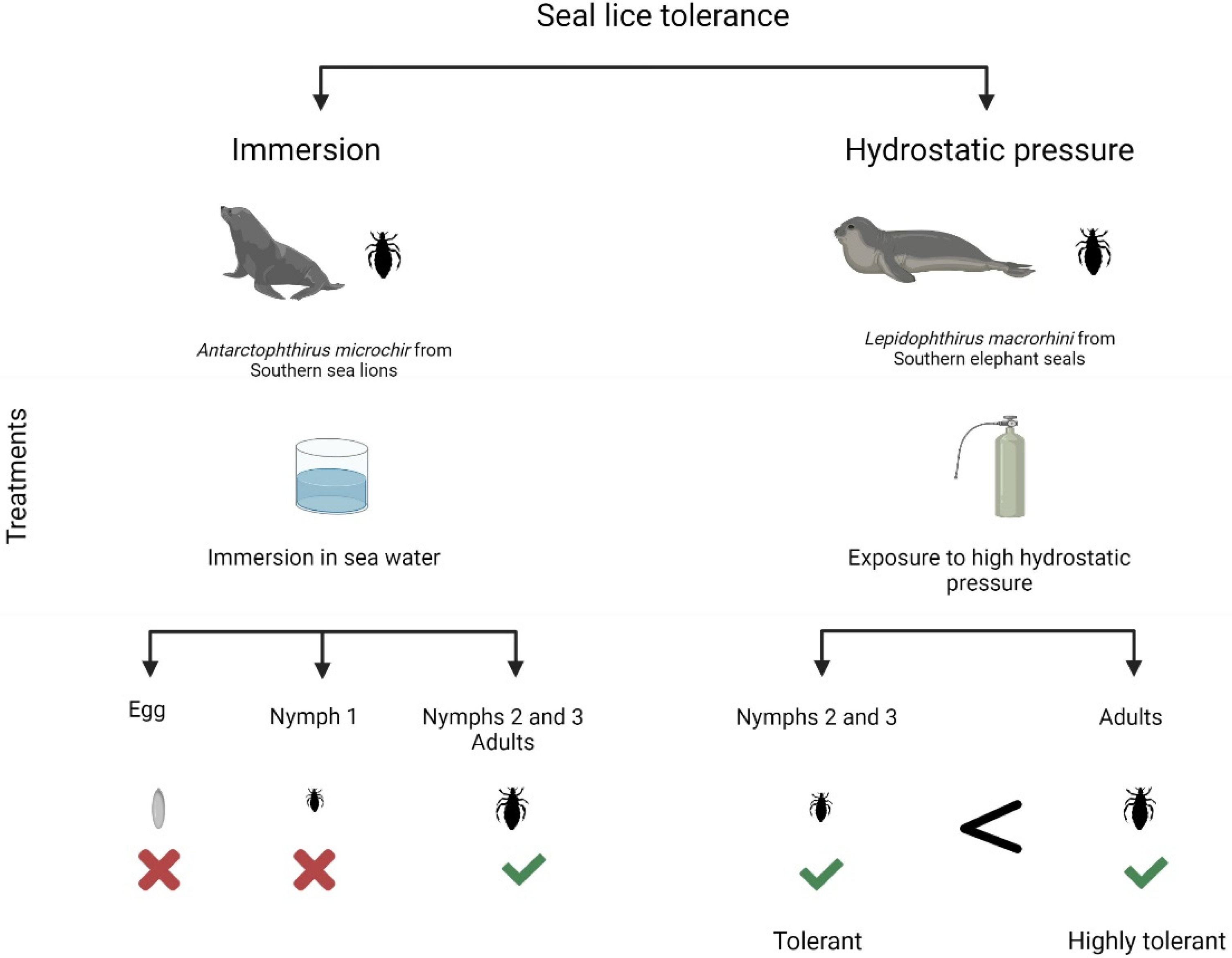
2.4. Tolerance to Immersion
2.6. Ecology
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/insects13010046
References
- Ruxton, G.D.; Humphries, S. Can ecological and evolutionary arguments solve the riddle of the missing marine insects? Mar. Ecol. 2008, 29, 72–75.
- Kim, K.C. Coevolution of Parasitic Arthropods and Mammals; Wiley: New York, NY, USA, 1985.
- Bush, A.O.; Fernández, J.C.; Esch, G.W.; Seed, J.R. Parasitism: The Diversity and Ecology of Animal Parasites; Cambridge University Press: Cambridge, UK, 2001.
- Durden, L.A.; Musser, G.G. The sucking lice (Insecta, Anoplura) of the world: A taxonomic checklist with records of mammalian hosts and geographical distributions. Bull. Am. Mus. Nat. 1994, 218, 1–90.
- Leonardi, M.S.; Palma, R.L. Review of the systematics, biology and ecology of lice from pinnipeds and river otters (Insecta: Phthiraptera: Anoplura: Echinophthiriidae). Zootaxa 2013, 3630, 445–466.
- Stewart, B. Diving behavior. In Encyclopedia of Marine Mammals; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 321–327.
- McIntyre, T.; de Bruyn, P.J.N.; Ansorge, I.J.; Bester, M.N.; Bornemann, H.; Plötz, J.; Tosh, C.A. A lifetime at depth: Vertical distribution of Southern elephant seals in the water column. Polar Biol. 2010, 33, 1037–1048.
- Teilmann, J.; Born, E.W.; Aquarone, M. Behaviour of ringed seals tagged with satellite transmitters in the North Water polynya. Can. J. Zool. 1999, 77, 1934–1946.
- Leonardi, M.S.; Virrueta Herrera, S.; Sweet, A.; Negrete, J.; Johnson, K.P. Phylogenomic analysis of seal lice reveals codivergence with their hosts. Syst. Entomol. 2019, 44, 699–708.
- Hassanin, A.; Veron, G.; Ropiquet, A.; Jansen van Vuuren, B.; Lécu, A.; Goodman, S.M.; Haider, J.; Nguyen, T.T. Evolutionary history of Carnivora (Mammalia, Laurasiatheria) inferred from mitochondrial genomes. PLoS ONE 2021, 16, e0240770.
- Arnason, U.; Gullberg, A.; Janke, A.; Kullberg, M.; Lehman, N.; Petrov, E.A.; Väinölä, R. Pinniped phylogeny and a new hypothesis for their origin and dispersal. Mol. Phylogenet. Evol. 2006, 41, 345–354.
- Aznar, F.J.; Balbuena, J.A.; Fernández, M.; Raga, J.A. Living Together: The parasites of marine mammals. In Marine Mammals; Evans, P.G.H., Raga, J.A., Eds.; Springer: Boston, MA, USA, 2002.
- Ménier, K. Origin and evolution of parasites in marine mammals: Pinnipedia as an example. Rev. Med. Vet. 2000, 151, 275–280.
- Leonardi, M.S. Clave para el reconocimiento de las especies de Echinophthiriidae (Phthiraptera: Anoplura) de Argentina y Antártida. Rev. Argent. Parasitol. 2014, 3, 24–30.
- Kim, K.C. Evolutionary parallelism in Anoplura and eutherian mammals. In Biosystematics of Haematophagous Insects, Systematics Association; Service, M.W., Ed.; Clarendon Press: Oxford, UK, 1988; pp. 91–114.
- Mehlhorn, B.; Mehlhorn, H.; Plötz, J. Light and scanning electron microscopical study on Antarctophthirus ogmorhini lice from the Antarctic seal Leptonychotes Weddellii. Parasitol. Res. 2002, 88, 651–660.
- Leonardi, M.S.; Crespo, E.A.; Raga, J.A.; Fernández, M. Scanning electron microscopy of Antarctophthirus microchir (Phthiraptera: Anoplura: Echinophthiriidae): Studying morphological adaptations to aquatic life. Micron 2012, 43, 929–936.
- Leonardi, M.S.; Krmpotic, C.; Barbeito, C.; Soto, F.; Loza, C.M.; Vera, R.; Negrete, J. I’ve got you under my skin: Inflammatory response to elephant seal’s lice. Med. Vet. Entomol. 2021, 35, 658–662.
- Kim, K.C. Ecology and morphological adaptation of the sucking lice (Anoplura, Echinophthiriidae) on the Northern Fur seal. Rapport et Procès verbaux des Réunions du conseil Permanent International pour lExploration de la Mer 1975, 169, 504–515.
- Leonardi, M.S.; Crespo, J.E.; Soto, F.A.; Vera, R.B.; Rua, J.C.; Lazzari, C.R. Under pressure: The extraordinary survival of seal lice in the deep sea. J. Exp. Biol. 2020, 223, jeb226811.
- Leonardi, M.S.; Crespo, E.A.; Raga, J.A.; Fernández, M. Redescription of Antarctophthirus microchir (Anoplura: Echinophthiriidae) from the South American sea lion, Otaria flavescens, from Patagonia, Argentina. J. Parasitol. 2009, 95, 1086–1092.
- Kim, K.C. The sucking lice (Anoplura: Echinophthiriidae) of the northern fur seal; descriptions and morphological adaptation. Ann. Entomol. Soc. Am. 1971, 64, 280–292.
- Murray, M.D. Insect parasite of marine bird and mammals. In Marine Insects; Cheng, L., Ed.; American Elsevier Publishing Company Inc.: New York, NY, USA, 1976; pp. 78–96.
- Leonardi, M.S.; Lazzari, C.R. Uncovering deep mysteries: The underwater life of an amphibious louse. J. Insect Physiol. 2014, 71, 164–169.
- Murray, M.D.; Smith, M.S.R.; Soucek, Z. Studies on the ectoparasites of seals and penguins. II. The ecology of the louse Antarctophthirus ogmorhini Enderlein on the Weddell seal, Leptonychotes weddelli Lesson. Aust. J. Zool. 1965, 13, 761–771.
- Leonardi, M.S.; Crespo, E.A.; Raga, J.A.; Aznar, F.J. Lousy mums: Patterns of vertical transmission of an amphibious louse. Parasitol. Res. 2013, 112, 3315–3323.
- Aznar, F.J.; Leonardi, M.S.; Berón-Vera, B.; Vales, D.G.; Ameghino, S.; Raga, J.A.; Crespo, E.A. Population dynamics of Antarctophthirus microchir (Anoplura: Echinophthiriidae) in pups from South American sea lion, Otaria flavescens, in Northern Patagonia. Parasitology 2009, 136, 293–303.
- Soto, F.A.; Klaich, M.J.; Negrete, J.; Leonardi, M.S. So happy together: Juvenile crabeater seal behavior improves lice transmission. Parasitol. Res. 2020, 119, 2059–2065.
- Murray, M.D.; Nicholls, D.G. Studies on the ectoparasites of seals and penguins I. The ecology of the louse Lepidophthirus macrorhini Enderlein on the Southern Elephant seal, Mirounga leonina. Aust. J. Zool. 1965, 13, 437–454.
- Murray, M.D. Ecology of the ectoparasites of seals and penguins. In Proceedings of the 1st SCAR Symposium of Antarctic Biology, Paris, France, 2–8 September 1962; Carrick, R., Prevost, J., Holdgate, M.W., Eds.; Hermann: Paris, France, 1964; pp. 241–245.
- Murray, M.D. Ecology of the louse Lepidophthirus macrorhini Enderlein 1904 on the elephant seal Mirounga leonina (L.). Nature 1958, 182, 404–405.
- Murray, M.D. Ectoparasites of Antarctic seals and birds. Jpn. Antarct. Res. Exped. Sci. Rep. 1967, 1, 185–191.
- Kim, K.C. Louse populations of the northern fur seal (Callorhinus ursinus). Am. J. Vet. Res. 1972, 33, 2027–2036.
- Leidenberger, S.; Harding, K.; Härkönen, T. Phocid seals, seal lice and heartworms: A terrestrial host-parasite system conveyed to the marine environment. Dis. Aquat. Org. 2007, 77, 235–253.
- Maddrell, S.H.P. Why are there no insects in the open sea? J. Exp. Biol. 1998, 201, 2461–2464.
- Harrison, J.F.; Woods, H.A.; Roberts, S.P. Ecological and Environmental Physiology of Insects; Oxford University Press: Oxford, UK, 2012.
