Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Allergy
Polycystic ovarian syndrome (PCOS) is a common endocrine disorder associated with hyperandrogenemia and failure of ovulation, which is often accompanied by metabolic abnormalities, including obesity, dyslipidemia, hyperinsulinemia, and insulin resistance. Research on proteins and peptides that play roles in metabolic regulation, which may be considered potential insulin resistance markers in some medical conditions, such as diabetes mellitus, obesity and PCOS.
- polycystic ovarian syndrome
- insulin resistance
- preptin
- myonectin
- gremlin
- omentin
- nesfatin
1. Introduction
To date, most research has been devoted to factors deriving directly from the white adipose tissue (WAT)—also known as adipokines—including adiponectin, resistin, leptin, visfatin, apelin, retinol-binding protein 4, and chemerin, to name but a few [5,6,7,8,9]. It has been shown that adipose tissue may function as an endocrine organ and that it secretes several adipokines, thus constituting a link between body mass excess and glucose level disturbances [7,10]. Adipokines may be synthesized in excess, or their expression may be diminished, in several conditions associated with insulin resistance, such as obesity, type 2 diabetes mellitus (T2DM), gestational diabetes mellitus (GDM), or polycystic ovarian syndrome (PCOS) [5,8].
PCOS is a frequent endocrine disorder associated with hyperinsulinemia and hyperandrogenemia, which is often accompanied by infertility and obesity [11]. Affected individuals are at increased risk of developing cardiovascular and metabolic diseases, as well as anxiety disorders [12].
The condition is most commonly diagnosed according to the Rotterdam criteria, when two out of the three following features occur (after the exclusion of related disorders): oligo/anovulation, clinical and/or biochemical hyperandrogenism, or polycystic ovaries on ultrasound [13,14].
The prevalence of PCOS has been estimated as 4–26% depending on the studied population (in terms of age, ethnicity, and so on) and applied criteria. It has been shown that adoption of the NIH/NICD guidelines may account for recognition of PCOS in 4–8% adult female patients, while this rate may be as high as 15–20%, when ESHRE recommendations are acknowledged [12].
Over the years, many hypotheses have been introduced, including genetic, epigenetic, and environmental factors [15]. The key pathogenetic factors include hyperandrogenemia, subclinical inflammation, and defective insulin signaling [16]. It has been evidenced that up to 50–80% of women with PCOS are diagnosed with insulin resistance [10,12,17]. Hyperinsulinemia is responsible for metabolic and cardiovascular complications [18], as well as decreasing sex hormone binding globulin (SHBG) production in the liver (resulting in an increase of free and bioactive androgen levels in the circulation) and potentiating the luteinizing hormone (LH)-dependent effect on the ovarian cells, leading to the enhanced synthesis of androgens [5]. Hyperandrogenemia in females further aggravates the course of metabolic complications [19,20].
Moreover, it has been shown that the presence of some bacterial species may lead to altered secretion of several peptides involved in the metabolic homeostasis and appetite regulation; for example, an increase of Bacteroides species has been shown to be associated with the dysregulation of ghrelin and peptide YY [23].
Last, but not least, in patients with PCOS, many studies assessing the levels of adipokines have been conducted [5] and it has been indicated that the dysregulation of such hormones leads to insulin resistance [6].
At present, there are emerging data regarding peptides/glycoproteins, synthesized in other tissues than WAT, which may also be involved in the pathomechanism of insulin resistance. Some of them have been investigated as potential insulin resistance biomarkers in PCOS [5]. Studying new possible markers may not only provide the insight into the intricate pathomechanism of PCOS, but also implicates that measurement of several target proteins that may provide useful information about the severity of the condition, possible complications, and prognoses, or which may serve as helpful tool for the early detection of metabolic complications. The analysis of such markers may also play a significant role in monitoring of the course of a disease [15].
3. Nesfatin-1
Nesfatin-1 (Figure 1) is an 82-amino acid neuropeptide derived from the post-translational processing of the N-terminal fragment of nucleobindin2 (NUCB2) [36], which was originally identified as an anorexigenic hypothalamic neuropeptide, a chronic intracerebroventricular injection of which reduced the food intake and decreased body weight and the amount of body fat [37]. Its expression has been detected in the areas responsible for appetite regulation, such as the arcuate, paraventricular, and supraoptic nuclei, as well as in the lateral hypothalamic area and zona incerta [37].
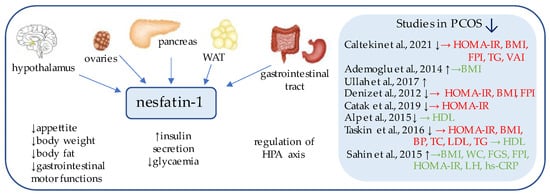
Figure 1. Role of nesfatin-1 in the pathogenesis of insulin resistance and PCOS. List of the studies assessing serum nesfatin level in the PCOS patients. ↑/↓ indicates whether concentration level of serum nesfatin was increased/decreased in PCOS individuals (p < 0.05); → (green)—indicates positive correlation between serum nesfatin and particular indicators; → (red)—indicates negative correlation between serum nesfatin and particular indicator; HOMA-IR—Homeostatic Model Assessment for Insulin Resistance; BMI—body mass index; FPI—fasting plasma insulin; TG—triglycerides; VAI—Visceral Adiposity Index; HDL—high density lipoprotein; BP—blood pressure; TC—total cholesterol; LDL—low-density lipoproteins; WC—waist circumference; FGS—Ferriman–Gallwey Score; LH—Luteinizing Hormone; hs-CRP—high-sensitivity C-Reactive Protein; WAT—white adipose tissue; HPA axis—Hypothalamic–Pituitary–Adrenal axis.
Subsequent studies have focused on the peripheral expression of nesfatin and proved its synthesis on mRNA and protein levels within the gut, pancreas, and white adipose tissue [37,38].
Interestingly, in the gastrointestinal tract, nesfatin-1 has been shown to be co-expressed in about 86% of x/A cells with the commonly known appetite-regulator ghrelin, and to a lesser extent, within the D cells with somatostatin and histamine-synthesizing enzyme histidine decarboxylase (HDC) [39,40]. Moreover, in the pancreas of rodents, beta cells co-localize insulin and pronesfatin immunoreactivity [41].
4. Preptin
Preptin (Figure 2) is a 34-amino acid peptide, a product of the same gene that encodes Insulin-like growth factor 2 (IGF II); more precisely, it corresponds to the Asp69–Leu102 sequence of the E-domain of its precursor, proinsulin-like growth factor II [56,57]. The peptide originated from the study of Buchanan et al., who purified it from secretory granules of cultured murine bTC6-F7 pancreatic b-cells [56], where preptin is co-secreted all together with insulin, amylin, and pancreastatin, and exhibits biological function as a glucose-mediated insulin secretion enhancer [1].
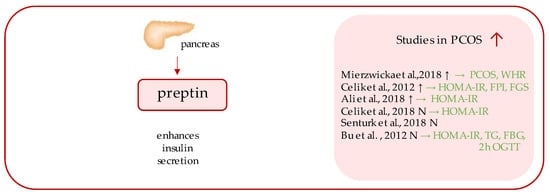
Figure 2. Role of preptin in the pathogenesis of insulin resistance and PCOS. List of the studies assessing serum nesfatin level in the PCOS patients. ↑/N indicates whether concentration level of serum preptin was increased/unchanged in PCOS individuals (p < 0.05); → (green)—indicates positive correlation between serum nesfatin and particular indicators; PCOS—polycystic ovarian syndrome; WHR—waist-to-hip ratio; HOMA-IR—Homeostatic Model Assessment for Insulin Resistance; FPI—fasting plasma insulin; FGS—Ferriman–Gallwey score; TG—triglycerides; FBG—fasting blood glucose; OGTT—oral glucose tolerance test.
Over the years, several studies aimed at measuring fasting preptin serum concentrations in females with PCOS were undertaken; however, the results of which remain inconsistent, as significantly higher serum preptin concentrations have been detected in some studies [26,58,59], while other studies have undermined this relationship [60,61,62].
5. Myonectin
Myonectin (Figure 3) belongs to the C1q/TNF-related proteins (CTRPs), constituting its most recent member, assigned as CTRP-15 [65,66]. CTRPs, due to presence of a C-terminal globular domain, with sequence homology to the immune complement protein C1q are further counted among the C1q protein family [66], together with a commonly known adipokine, adiponectin, which is a well-established insulin-sensitizing hormone [67], the diminished levels of which reflect insulin resistance, which has been repeatedly demonstrated in PCOS individuals [68,69]. Unlike the other CTRPs, which are widely expressed in many tissues, most predominantly in adipocytes [66], myonectin is an example of a myokine, with its highest expression occurring within the skeletal muscles [65]. Initially, its function was linked to the promotion of fatty acid uptake by adipocytes and hepatocytes, consequently leading to a reduction of serum free fatty levels [65]. Importantly, it has been shown to be up-regulated after feeding and physical exercise [65].
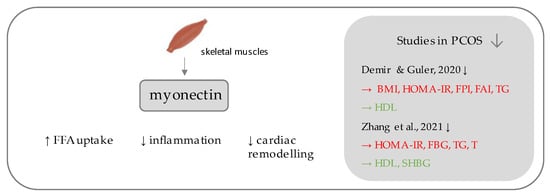
Figure 3. Role of myonectin in the pathogenesis of insulin resistance and PCOS. List of the studies assessing serum nesfatin level in the PCOS patients. ↓ indicates that concentration level of serum myonectin was decreased in PCOS individuals (p < 0.05); → (green)—indicates positive correlation between serum myonectin and particular indicators; → (red)—indicates negative correlation between serum myonectin and particular indicator; BMI—Body Mass Index; HOMA-IR—Homeostatic Model Assessment for Insulin Resistance; FPI—fasting plasma insulin; FAI—free androgen index; TG—triglycerides; HDL—high density lipoproteins; FBG—fasting blood glucose; T—testosterone; SHBG—sex hormone binding globulin.
6. Omentin-1
Omentin-1 (Figure 4) is a 296-amino acid glycoprotein, a major form of the circulating omentins in human plasma, products of the gene localized within the 1q22–q23 chromosomal region which has been linked to T2DM in various populations [9,81]. It belongs to the adipokines, the expression of which occurs largely in the visceral (omental) and, to a twenty times lesser extent, in the subcutaneous adipose tissue and is a factor detectable in human serum [82,83]. Indeed, its function relies on increasing insulin-mediated glucose uptake by activating the protein kinase B (PKB or Akt) pathway, and decreased serum concentrations have been detected in individuals with obesity and T2DM [82,85]. It is predominantly positively related to adiponectin and negatively to BMI, leptin, and insulin resistance [85]. Therapies based on body weight reduction have been shown to contribute to elevation of its serum concentration [86,87]. Beside its anti-diabetic properties, omentin also decreases cardiovascular risk and preserves anti-inflammatory and antiatherogenic functions, through vasodilation of blood vessels, attenuation of C-reactive protein-induced angiogenesis, and reduction of tumor necrosis factor-α (TNFα)-induced inflammation in the endothelium and vascular smooth cells [88,89,90].
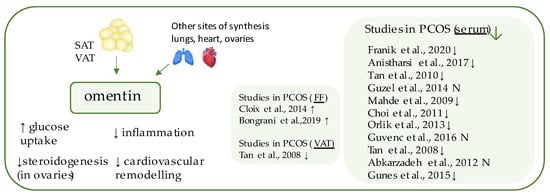
Figure 4. Role of omentin in the pathogenesis of insulin resistance and PCOS. List of the studies assessing nesfatin level in serum, follicular fluid (FF), and visceral adipose tissue (VAT) of the patients with PCOS. ↓/N indicates that concentration level of omentin was decreased/unchanged in PCOS individuals (p < 0.05).
7. Gremlin
Gremlins (Figure 5) are peptides belonging to DAN family, which function as extracellular antagonists of bone morphogenetic proteins (BMPs), especially BMP2 and BMP4 [101,102]. BMPs are highly conserved proteins, which exhibit transforming growth factor β (TGF-β) activity and regulate cell differentiation during embryogenesis and later stages of life [103]. Gremlins (1 and 2) neutralize those ligands by binding to them and preventing their interaction with receptors and signaling [102].
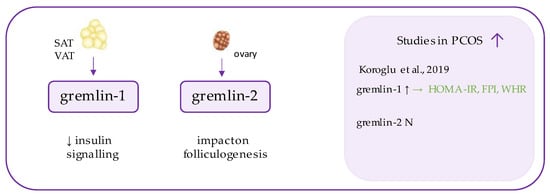
Figure 5. Role of gremlin in the pathogenesis of insulin resistance and PCOS. List of the studies assessing gremlins level in serum of the patients with PCOS. ↑/N indicates whether concentration level of gremlin was increased/unchanged in PCOS individuals (p < 0.05); → (green)—indicates positive correlation between serum omentin and particular indicators; HOMA-IR—Homeostatic Model Assessment for Insulin Resistance, FPI—fasting plasma insulin, WHR—waist-to-hip ratio.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph19042099
This entry is offline, you can click here to edit this entry!
