Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The visible and near-infrared wavelengths can affect bacterial growth. Like in eukaryotic cells also in bacteria, photobiomodulation can affect cellular metabolism, homeostasis, defence to stress, and life-and-death mechanisms. Light-bacteria interaction for microbiota management can represent a supportive medical approach in health and illness patients.
- gum disease
- laser therapy
- light therapy
- microbiome
- prokaryote
- periodontal disease
- oral infection
1. Photobiomodulation
Photobiomodulation (PBM), previously known as Low-Level Laser Therapy (LLLT), causes cell manipulation by a photon energy transfer employing non-ionizing light sources in the visible and infrared spectrum, including lasers, light-emitting diodes (LEDs), and broadband light [1]. This therapy, based on non-ablative energies, is a non-thermal process, which involves endogenous photo-acceptors eliciting photophysical (i.e., linear and nonlinear) and photochemical events at various biological scales [1].
Although humans and animals have not utilized light as their primary energy source, many molecules involved in their physiology have retained their primordial photoacceptive properties, inserting the energized molecules into the first proto-cells and then into prokaryotes outer membrane during their evolution (Figure 1). Afterward, these molecules were inserted into the inner membrane of the mitochondria in free eukaryotic cells, thus becoming part of their physiology. In many cases, the energized molecules gradually lost the possibility to utilize direct light interaction. However, photoacceptors can be modulated by PBM at specific wavelengths of light, thus influencing cell physiology.

Figure 1. Parallel origin and evolution of life and photoacceptors. The conversion of physical energy (sunlight, geothermal events, lightning) in mechanical work led to the organization of complex molecules and polymers (A). Copper (Cu), iron (Fe), and manganese (Mn) could have been excited by the absorption of photon energy. This event allowed the generation of structures with increasing complexity, which after incorporation of those minerals, worked as ancient components of the metabolic system. These primordial cytochromes, porphyrins, chlorophylls, pigments, flavins, pteridines inherited the ability to interact with light and spontaneously aggregated through fatty acid in microspheres (B). Peptide-nucleic acid formation also occurred. A first proto-cell formed, which was able to produce energy (ATP) through the photocatalytic phosphorylation of ADP and make copies of itself thanks to the generation of information-containing molecules. Eukaryotic cells arose through a first-symbiosis between an H2-dependent methanogenic archaeon and a facultative anaerobic alpha-proteobacterium, which became the “universal” non-obligatory anaerobic mitochondrion and contributed to the nucleus formation (C). Moreover, mitosomes and hydrogenosomes evolved from this mitochondrion based on the ecological niche colonized by the host. A second symbiosis between the facultative anaerobic first-eukaryotic cell and a cyanobacterium (D) led to an ancestral plant cell, which was followed by three plastid lineages: chloroplastida, glaucophytes, and rhodophytes. Therefore, metals and molecules that are able to be energized by photons have been transmitted through evolution from the life origin into the primordial broth to prokaryotic and eukaryotic cells, where are involved in their metabolism and physiology.
The PBM primary mechanisms are based on the Grotthuss–Draper law (principle of photochemical activation), which states that only the light absorbed by a system can bring about a photochemical change[2].
Pastore et al. [3] showed that COX (respiratory complex IV) acts as a photoacceptor at 632.8 nm due to two heme A moieties and two copper centers. On the other hand, COX also displays absorption peaks at 450, 620–680, and 760–895 nm [4]. Moreover, it was shown that 808 and 980 nm selectively stimulated complex IV and, in part, complex III, which contains a cytochrome b subunit with two heme moieties, a cytochrome c1 subunit with one heme group, and a Rieske protein subunit (UQCRFS1) with a 2Fe-2S cluster[5][6][7] . Conversely, 1064 nm wavelength affect complex I (with eight 2Fe-2S clusters), in addition to complexes III and IV . The extrinsic mitochondrial membrane complex II (with a heme B prosthetic group) does not seem receptive to photons at these wavelengths [4][5][6][7].
The visible 400–500 nm wavelengths excite flavins and flavoproteins[8]. Thus, light could act on different cell pigments and respiratory complexes I and II[9]. In addition, porphyrins, heterocyclic organic compounds complexed to hemoglobin, CYP enzymes, and complex IV possess the ability to absorb light at 400–420 nm [10] and 450 nm [11]. Heme-containing protein and di-nitrosyl iron complexes form complexes with NO (i.e., NO-hemoglobin) as well as the thiol groups (i.e., S-nitrosothiols), and light may induce NO release from a variety of cellular sources [12]. Lastly, near-infrared light seems to excite water, affecting temperature-gated calcium (Ca2+) ion channels [13] and lipids that show a mild but significant absorption peak in the range of 900–1000 nm [14]. Meanwhile, visible light modulates the structure and activities of the opsin proteins family, which are involved in cellular pathways of different cell types[15].
The primary PBM targets are linked to the endogenous release of reactive oxygen species (ROS) and NO, ATP production, and modulation of Ca2+ fluxes and redox homeostasis, which can play a key role in cell proliferation, growth, and apoptosis[16][17][18]. Therefore, PBM therapy seems to support treatments in many medical and veterinary areas to restore cell dysfunction and promote recovery from illness [19][20][21]22][23][24][25]. PBM therapy has been recommended unequivocally for oral mucositis prevention in patients treated with chemotherapy by the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology [26]. However, concerns have been raised regarding the potential stimulatory effect on existing malignant or pre-malignant cells and induction of therapeutic resistance[27][28][29]. Chemotherapy also supports the progression of gingivitis and periodontitis by bacterial pathogen growth [30]. Therefore, because of the ubiquitous presence of the primary targets of PBM in all kingdoms of life[20] , the modulatory effect of light therapy on the prokaryotic communities needs attention in view of the role of microbiota in human health. In that regard, Liebert and colleagues recently introduced the term ‘photobiomics’ to represent the PBM effects on microorganisms [31].
2. The Oral Microbiota in Health and Disease
The neo-Darwinian evolution has worked to distance and increase the complexity among the protocell, bacteria, and mammals. However, bacteria and humans have organized a coevolutionary and mutualistic relationship for billions of years. Therefore, a modern vision in medicine considers the human body as a complex assemblage of eukaryotic and prokaryotic cells organized into functional organs, tissues, and cellular communities. Ninety percent of the cells in and on the human body are microbial cells and, despite the presence of viruses, archaea, yeast, and protozoa, the most represented community is bacteria. In the human body, the entire microbial community, called microbiota, is principally formed by four phyla—Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes—which colonize the oral cavity, esophagus, skin, vagina, and gut [32]. Microbiota colonization occurs and is modified quickly in the early years of life, while in an adult, it remains relatively stable and is unique to each person [33]. However, the microbiota is a living ecosystem undergoing growth rate fluctuations and survival because of changes in diet, vigorous cleaning and disinfection, lifestyle, drugs (i.e., antibiotics), and diseases[32][33]. The constitutional microbiota may re-emerge when the original conditions resume [32][33]. The human microbiota consists of a core part, relatively constant in all the individuals, and a variable part associated with the individual case history. During its life cycle, the microorganisms belonging to the microbiota interact with each other and the human host cells through intraspecies or interspecies communication. Bacteria can modulate tissue signaling pathways and immune cell responses. Moreover, they produce vitamins (i.e., cobalamin) and bacteriocins, molecules able to inhibit or kill bacteria. In other words, the microbiota causes beneficial or detrimental changes in the host [34]. Indeed, dysbiosis—loss of balance within a human-associated microbial community—is associated with several pathological conditions, such as insulin resistance in patients with type 2 diabetes, esophagitis and Barrett’s esophagus, ulcers, inflammatory bowel disorder (Crohn’s disease), recurrent abdominal pain, vaginitis, arthritis, autism, neurodegenerative diseases, cancers, collateral periodontitis, and macular degeneration[32][35][36][37]. Microbiome characterization offers an opportunity for innovative diagnostic biomarkers and therapy.
The oral cavity harbors over 700 species of bacteria and represent the second-largest heterogeneous microbiota of the human body, after the gut [38]. Bacteria can colonize two different surfaces in the oral cavity: the hard tissue of the teeth and the soft tissues of the oral mucosa of the tongue, cheeks, gingival sulcus, tonsils, and palate as well as saliva. Deo and Deshmukh [38] showed that the principal bacterial genera found in the healthy oral cavity are:
-
gram-positive: cocci—Abiotrophia, Peptostreptococcus, Streptococcus, and Stomatococcus; rods—Actinomyces, Bifidobacterium, Corynebacterium, Eubacterium, Lactobacillus, Propionibacterium, Pseudoramibacter, and Rothia;
-
gram-negative: cocci—Moraxella, Neisseria, and Veillonella; rods—Campylobacter, Capnocytophaga, Desulfobacter, Desulfovibrio, Eikenella, Fusobacterium, Hemophilus, Leptotrichia, Prevotella, Selemonas, Simonsiella, Treponema, and Wolinella;
-
the uncultured divisions GN02, SR1, and TM7 [39].
For further information, please consult the Human Oral Microbiome database website www.homd.org (30 December 2021) and the NIH Human Microbiome Project https://www.hmpdacc.org/ (30 December 2021). This commensal microbiota plays a key role in maintaining oral and systemic health [40] through bacteriocin and biofilm formation against pathogens (colonization resistance).
Dysbiosis paves the way for opportunistic pathogens such as Candida spp. and Staphylococcus spp.[38] . In addition, this condition exhibits cariogenic properties by Streptococcus mutans, Actinomyces naeslundii, Propionibacterium spp., and Lactobacillus spp., or to induce periodontitis and halitosis by Streptococcus salivarius. Periodontitis is also favored by the colonization of the periodontal pocket and its infection with Porphyromonas gingivalis, Treponema denticola, Anaeroglobus geminatus, Tannerella forsythia, Filifactor alocis, Eubacterium saphenum, Prevotella denticola, Prevotella intermedia, and Porphyromonas endodontalis [39][40]. Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis, and Staphylococcus aureus were found to colonize the oral cavity of hospitalized patients, and the presence of Helicobacter pylori in dental plaques was directly associated with gastric infection [41]. Dentine lesions facilitate anaerobic proteolytic bacteria and enterococci. Lastly, P. gingivalis and Fusobacterium nucleatum may provoke oro-digestive cancers and oral squamous cell carcinoma[40][42]. Oral infection can gain access to the bloodstream and cause infectious endocarditis; brain, kidney, and liver abscesses; rheumatoid arthritis; Alzheimer’s disease and dementia; as well as pregnancy-related complications [39][40]. As a result, the human microbiota is an emerging target for the development of a modern therapeutic approach to several human diseases.
3. Photobiomodulation on Bacterial Microbiota
3.1. Evidence-Based Literature
In the past, most infections of odontogenic origin have been managed by dentists through antibiotics therapy and prophylaxis[43] . However, the ability of bacteria to survive in drug concentrations that should kill or inhibit them, and their routinely indiscriminate prescription, has allowed antibiotic resistance to occur[44][45]. Despite, in some cases, the prescription of antibiotics being essential, the risk of antibiotic toxicity and allergies can limit their applicability. Thus, the light could be a suitable alternative cure supporting oral infection prevention and cure. Indeed, in nature, solar radiation is shown to select for pigmented bacteria [46]. Culture-to-culture physical interactions mediated by biophoton visible and near-infrared light emission were also preliminarily described in E. coli cultures [47]. On the other hand, UV irradiation is well-known to photo-destroy bacteria. Unfortunately, even minimal overexposure to UV is dangerous to healthy tissue [48]. Additionally, applying local or systemic exogenous photosensitizers, inappropriate cells can be destroyed by specific light wavelengths. This application is known as photodynamic therapy[49].
It previously discussed how PBM, through the interaction of visible and near-infrared light with endogenous photoacceptors, can positively affect normal eukaryotic cell metabolism and support recovery from disease. Therefore, according to the PBM mechanism of action and the prevalence of molecular photoacceptors in all life forms, the PBM therapy could also affect bacteria cells.
The literature about bacteria and photobiomodulation discussed herein was screened through keywords such as bacteria, microbiota, microbiome, low-level laser therapy, light therapy, and photobiomodulation on PubMed and Scholar databases. Articles were also selected from the references of papers reviewed.
Bicknell et al. [100] showed that PBM at 660 and 808 nm influenced the gut microbiota of mice. Infrared light particularly affected Allobaculum cells, which increased their growth. Using the same wavelength, Thomé Lima and collaborators suggested that PBM can improve mouse wound healing by killing or inhibiting Pantoea agglomerans bacterium [101]. Similarly, faster healing and regeneration were observed by Amaroli and colleagues in Dendrobaena veneta after irradiation with 808 nm PBM, where the therapy significantly decreased bacterial load [102].
The PBM also seems to influence the bacteria cell cycle that regularly or occasionally forms the oral microbiota in healthy and/or ill patients . Indeed, literature shows that P. gingivalis, F. nucleatum, S. mutans, and E. faecalis exposed to visible light at wavelengths of 400–500 nm, at power densities between 0.26 and 1.14 W/cm2 (60–180 s), manifested a phototoxic effect [103]. P. gingivalis and F. nucleatum were more sensible and exhibited effects with the minimal fluences of 16–39 J/cm2, while S. mutans and E. faecalis needed 159–212 J/cm2. The effect is not due to an indirect medium modification nor its dangerous increase in temperature. However, an infrared diode laser wavelength of 830 nm did not affect the cells [103]. In the same way, Henry et al. [104], through 488–514 nm laser lights, but lower fluences of 4.2 and 21 J/cm2, exerted a drastic phototoxic effect on P. intermedia. Only a mild effect was observed on P. gingivalis, while P. denticola and P. endodontalis were not affected.
Because of their feature of black-pigmented bacteria, authors concluded that the nature of the metabolic pathways for porphyrin synthesis could protect P. denticola and P. endodontalis but made P. intermedia more susceptible to damage from these wavelengths. A better effect of the lower wavelengths than that at 800–900 nm was also described by Nussbaum et al. [105] when 0.015 W/cm2 and 1–50 J/cm2 were irradiated in continuous wave (CW) mode on P. aeruginosa, E. coli, and S. aureus. Specifically, 630 nm appeared most associated with bacterial inhibition compared to 810 and 905 nm. Interestingly, E. coli growth was inhibited by 630 nm and 1 J/cm2, but significantly increased at 810 nm and 20 J/cm2. However, the same team, in a comparative study between CW or frequency-modulated light mode of irradiation of 810 nm (0.015 W/cm2; 1–50 J/cm2; 26, 292, 1000, or 3800 Hz) showed that laser-mediated growth of S. aureus and E. coli was dependent on pulse frequency [106]. In addition, P. aeruginosa growth increased up to 192%, using 1000–3800 Hz, whereas 26–292 Hz pulsing irradiation produced only a growth trend. All bacteria increased proliferation after irradiation with 810 nm in CW mode.
Different evidence concerning the near-infrared wavelengths and the CW mode of irradiation was described by de Sousa et al. [107] on S. aureus, E. coli, and P. aeruginosa and Dixit et al. [108] on bacterial strains of P. aeruginosa, E. coli, E. faecalis, S. epidermidis, Streptococcus pyogenes, Shigella, Salmonella sp., Staphylococcus saprophyticus, Salmonella typhi, S. epidermidis, S. aureus, and Klebsiella pneumoniae. In detail, P. aeruginosa was inhibited at the wavelengths of 660, 830, and 904 at a fluence of 24 J/cm2. E. coli had similar growth inhibition at a wavelength of 830 nm at fluences of 3, 6, 12, and 24 J/cm2. At wavelengths of 660 and 904 nm, growth inhibition was only observed at fluences of 12 J/cm2 and 18 J/cm2, respectively [107]. Meanwhile, at 810 nm and laser fluences of 13 J/cm2, 18 J/cm2, and 30 J/cm2 had effectiveness in the treatment of Gram-negative and Gram-positive bacteria [108], and the effects were higher in Gram-positive.
De Sousa et al. [109] also reported that 830 and 904 nm wavelengths at a fluence of 3 J/cm2 significantly induced topographical changes of the S. aureus cell structure. Additionally, S. aureus, P. aeruginosa, and E. coli growth were inhibited at fluences >6 J/cm2 when irradiated with a 450 nm laser light [110].
Near-infrared laser light of 810 nm for 30 s in two cycles with 1.5 W and 1 W exerted an antibacterial effect against three cariogenic bacteria, such as S. mutans, Lactobacillus casei, and Actinomyces naeslundii [111]. S. mutans irradiation at 780 nm, 400 mW, 5–20 J/cm2, and 250–1000 s [112] decreased the proliferation in a dose-dependent manner.
Plavskii et al. [113] showed that laser radiation of 405 and 445 nm causes growth inhibition in S. aureus and E. coli. Similarly, blue light wavelengths affected Prevotella spp. [114], P. gingivalis [115], and P. aeruginosa [116], but the effect was more evident in Prevotella spp. Based on these data, the blue spectral region radiation, like that previously shown with cyan light, may act through a sensitizing effect of endogenous porphyrins and flavin-type capable of inducing reactive oxygen species generation. However, S. mutans generally exhibited sensitivity to PBM therapy and was barely affected by blue light when grown in an anaerobiotic environment. Over the range of blue light, 400–410 nm (15 J/cm2) but not 430 nm significantly suppressed P. gingivalis growth [117]. S. aureus cell division has been affected and inhibited by irradiation with 514, 532, and 633 nm [118], while P. aeruginosa was stimulated.
The suggestions derived by the Karu experiments indicate support of E. coli cell proliferation after irradiation with a wide range of wavelengths and doses [119,120,121]. Furthermore, Bertoloni et al. [122] described the stimulatory effect of 632.8 nm of light (4 J/cm2) on E. coli, since the cells exhibited enhanced cell metabolism and intensified synthesis of cytoplasmic membrane proteins, increased cell volume, and ribosomal content. Dadras et al. [118], in contrast to its results on S. aureus, observed that P. aeruginosa increased the cell multiplication when exposed to PBM at 514, 532, and 633 nm.
Lastly, in vivo experiments on rats [123] affected by periodontitis induced by 5-fluorouracil chemotherapy describe the positive effect of scaling and root planing associated with multiple PBM sessions (660 nm; 0.035 W; 4.2 J; 120 s) on periodontitis recovery. The effect involved the response to the therapy of the oral microbiota such as Aggregatibacter actinomycetemcomitans, P. gingivalis, Prevotella nigrescens, and F. nucleatum.
The absence of comparative and exhaustive studies does not allow extrapolating reliable clinical approaches but only therapeutic indications.
The comparison of research by de Sousa et al. [110] and Nussbaum et al. [105,106] suggest some indications about bacteria-photobiomodulation interactions. Staphylococcus aureus, P. aeruginosa, and E. coli were sensitive to 450 nm irradiation using different intensities, although the effect did not appear strictly correlated to fluences [110], except for E. coli that was not affected by 24 J/cm2. Conversely, the wavelengths of 630, 660, 810, and 905 nm and a wide range of fluences discordantly impacted the bacteria cell growth [105].
For instance, the 630 nm through 1 J/cm2 drastically inhibited P. aeruginosa growth, but 2 J/cm2 increased it, and the effect took turns at 5, 10, 20, and 50 J/cm2. However, at 810 nm, growth increment was observed after irradiation with 1 and 2 J/cm2, while the other fluences (5–50 J/cm2) inhibited it with different intensities.
In general, the bacteria-photobiomodulation interaction seems not to follow the hormetic behavior of eukaryotic cells, but reflects the windows-effects shown in the previous studies on mitochondria [56].
3.2. Possible Mechanism of Action
Visible and near-infrared light affects the bacteria cell cycle through primary interactions on photoacceptive molecules and pigments (Figure 2). The PBM exerts a direct action when its targets are into the bacterial cell or released in the microorganism colony. However, bacteria can be sensitive to indirect effects exerted by tissues and cells surrounding the bacteria. In the first case, PBM can directly modulate the cell metabolism and defenses through the photo-energization and the non-thermal effect of light on photoacceptors (i.e., cytochromes, flavins, iron-proteins). Conversely, light interaction may also occur through the energization of pigments of endogenous nature, followed by thermal or like-photodynamic effects. In both cases, PBM can determine cell fate [98].
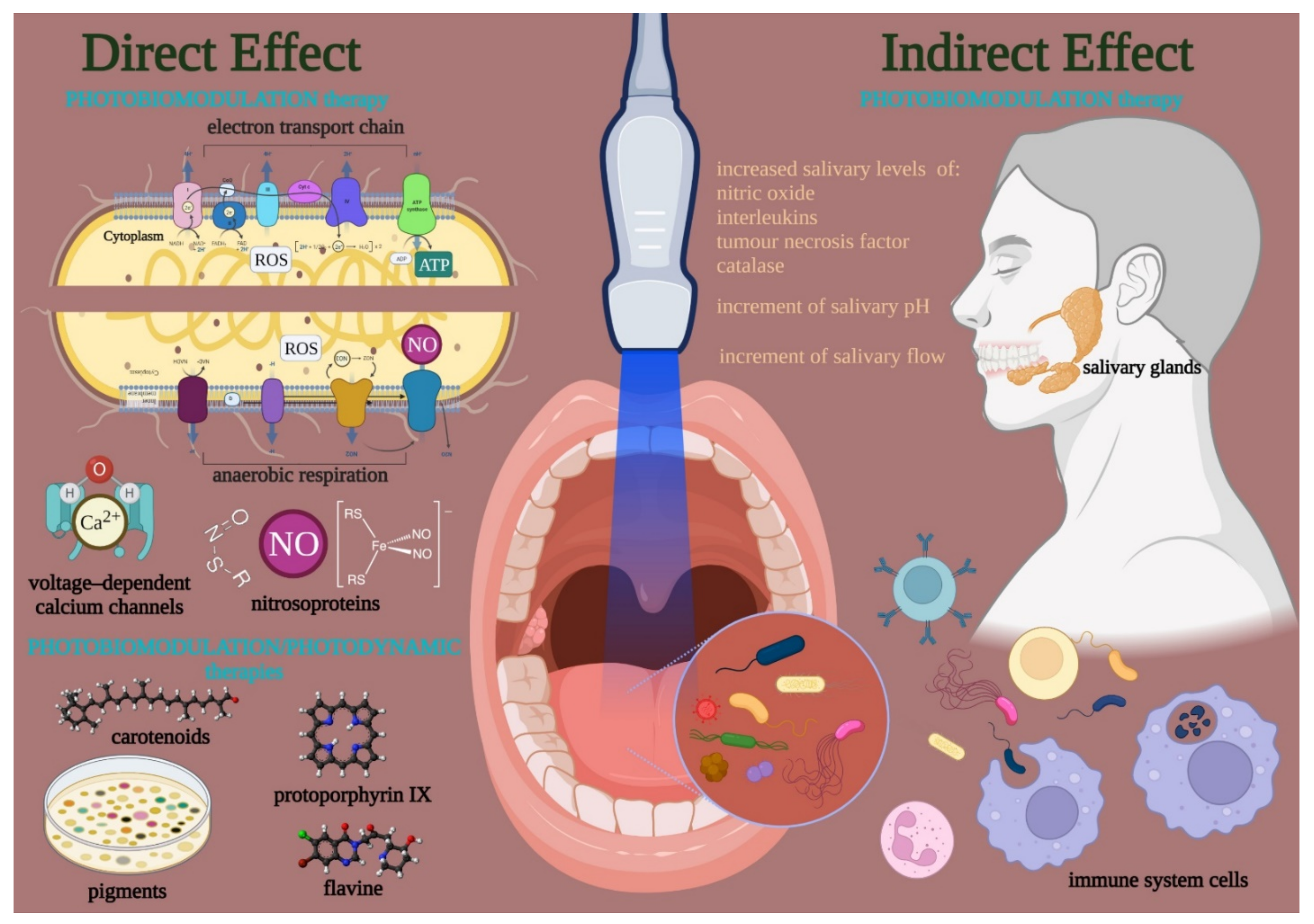
Figure 2. Visible and near-infrared light can modulate the bacteria cell cycle through primary interactions on photoacceptive molecules and pigment targets. A direct effect occurred when the endogenous targets are kept on/in the cell or released in the colony. Conversely, targets in tissues and cells surrounding the bacteria can lead to an indirect effect. The direct effects support a PBM in the strict sense, which modulates the cell metabolism and defense through the photo-energization and the non-thermal effect of light on photoacceptors such as cytochrome, flavins, iron-proteins of the electron transport chain or the anaerobic respiration, nitroso-protein, and voltage-dependent calcium (Ca2+) channels; the interaction followed by ATP and reactive oxygen species (ROS) production, nitric oxide (NO) release, and calcium homeostasis modulation. On the other hand, a PBM in a broad sense like a photodynamic effect may occur through the interaction of photons with pigments (i.e., carotenoids, porphyrins) flavins of endogenous nature, which is followed by thermal or oxidative cell damage. In both cases, PBM can modulate the life-and-death mechanisms of the bacteria. However, the PBM may also affect the oral tissue surrounding bacteria and modulate the quality and amount of salivary gland activity and the immune system’s behavior.
Literature shows that PBM acts on Gram-negative and Gram-positive bacteria without distinction [103,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123]. Indeed, Dixit et al. [108] described a greater effect of an 810 nm laser irradiation on Gram-positive than Gram-negative. However, the same wavelength similarly affected the two groups when irradiated on E. coli, P. aeruginosa, and S. aureus . Conversely, 400–500 nm prevalently inhibited the cell growth of P. gingivalis and F. nucleatum (Gram-negative) with respect to S. mutans and E. faecalis (Gram-positive) [103]. Basically, authors [107] showed that the difference in the PBM therapy efficacy was more correlated with the organism than the group. The wavelengths used in the PBM experiments did not seem to interact with the peptidoglycans or bacterial cell wall lipids. However, these molecules could be sensitive to wavelengths higher than 900 nm, such as 980 nm and 1064 nm [64], since they display some absorption peaks in this spectrum range as well as infrared. The aerobic/anaerobic-facultative or anaerobic metabolism is likewise non-discriminatory and, in both, PBM may occur. Unfortunately, a comparison with strictly aerobic bacteria was not investigated in depth. However, it should be noted that S. mutans is sensitive to PBM therapies in a wide range of wavelengths [103,111,112], but the trend changed when the experiments were performed in an anaerobic environment with respect to aerobic growth conditions.
Generally, the effect is correlated with wavelengths, dose, irradiation mode (CW or pulsed), and bacteria strain. For instance, E. coli growth can be inhibited or stimulated by different wavelengths, although CW seems the better irradiation mode with respect to pulsed [106,109,110,119,120,121,122]. Visible light at the wavelengths of 514, 532, and 633 nm induced a proliferative effect on P. aeruginosa but inhibited S. aureus, even if both are aerobic/facultative-anaerobic bacteria [118].
Like in the eukaryotic cell, through the modulation of mitochondria metabolism, bacteria may also be affected by PBM with visible and near-infrared laser light.
Bacterial metabolic conditions, growth phase, and condition of bacterial culture (rich medium or poor medium) seem to affect the PBM effect [125]. Fukui et al. [117] suggested that PBM irradiation might affect P. gingivalis metabolism as well as growth, and Basso et al. [112] showed that the induction of cell death in S. aureus is mediated by inhibition of its metabolism.
Indeed, the bacteria ETC expresses protein complexes and molecules transferring electrons from an electron donor to an electron acceptor, and, even if the model can be different according to bacterial species, it always leads to ATP production in aerobic conditions [126]. Alongside the water-soluble cytochromes working as electron shuttles, which seem not involved in the light interaction [55], other complexes and cytochromes exhibit macromolecular structures embedded into the cell membrane and show similarity with mitochondria complex I, III, and IV. Mitochondria complex IV has many peaks of absorption from 450 to 900 nm [53] and, along with complex III [55,56,57] and I [57], can be modulated by PBM at 810, 980, 1064 nm of wavelengths. In addition, the succinate-quinone oxidoreductase, an analog of mitochondria complex II [126], could be stimulated by visible light through its flavoproteins. In other words, the PBM can influence ATP production in bacteria, as already observed in eukaryotic cells. However, the ETC is one of the main sites of ROS production in bacterial cells [126,127]. Thus, like in normal and cancerous eukaryotic cells, the different PBM effects on bacterial growth can be correlated to the modulation of energy metabolism and the balance between oxidative stress production and antioxidant defenses. Lushchak reviewed the role of oxidative stress and the mechanisms of protection against it in bacteria [126]. It was showed that ROS-induced damage occurs mainly in sites containing iron and copper, causing oxidation of thiol groups of cysteine and methionine, imidazole ring of a histidine, and the rings of tyrosine, phenylalanine, and tryptophan. Additionally, ROS also interacts with DNA and polyunsaturated fatty acids, provoking damage on genome and lipid structures. However, cells display several antioxidant defenses to counteract ROS production. For example, catalase plays a pivotal role in protecting bacteria from oxidative stress, and its iron-containing heme groups can be a target of PBM [127]. It was recently showed that 810 nm PBM may reduce the catalase activity in a head and neck squamous carcinoma cellular model, determining an unbalance between oxidative stress production and the antioxidant defenses and stimulating the pro-apoptotic cellular pathways [77].
Conversely, strictly anaerobic bacteria seem to not need respiratory cytochrome oxidases. Nevertheless, functional cytochrome bd-type oxidases with iron groups have been discovered in strictly anaerobic bacteria [128], and heme-proteins play a role in the anaerobic bacterial formation of ATP [37,38,42]. Thus, a similar effect may be assumed on bacteria. Indeed, Lubart et al. [98] reviewed the effect of laser light on bacteria, pointing out ROS production through the effects of PBM on the prokaryote metabolism. They showed that, like in eukaryotes cells, phototoxic effects followed the induction of high amounts of ROS, while low amounts of them promoted proliferation.
Verkhratsky et al. [129] recently reviewed the evolution of Ca2+ signaling and Ca2+ channels, and they point out that the voltage-dependence of Ca2+ channels in E. coli resembles that of low-voltage-activated (T) Ca2+ channels in eukaryotes. Therefore, like that observed on RBL-2H3 mast cells [130], the PBM could similarly interfere with the bacterial calcium homeostasis and the related processes.
Therefore, bacteria could display primary targets for the interaction with visible and near-infrared light and be affected directly by PBM. However, data are scanty, particularly focused on bacteria responsible for oral cavity disease, and there are no relevant community studies on commensal and pathogens. In addition, the studies are prevalently in vitro and with observational conclusions (growth-stimulating, bacteriostatic, bactericidal effects). Therefore, achieving unequivocal conclusions is nowadays impossible.
Bacteria can also produce endogenous pigments or release them into the biofilm [131,132]. Moreover, in some cases, the association between pigmented and non-pigmented bacteria was observed since, as mentioned above, pigments can generate indirect PBM effects. Bacteria can be killed by light according to the pigment produced and the wavelengths employed [114,123]. Specifically, the light energy absorption can generate a thermal increase [133] incompatible with life [98,132], or the energized pigments could increase ROS formation through photodynamic therapy pathways [113]. Therefore, the pigmented bacteria can be killed by light at a low-level dose reliable for PBM, and non-pigmented bacteria associated with the colony can be involved in this lethal effect. This could explain the PBM’s negative effects on Porphyromonas spp. and Prevotells spp. at the wavelength of 400, 410 nm, or in the range of 400–500 nm, respectively, but not at 810 nm [103,117]. On the other hand, both bacteria produce black pigment due to the accumulation of Fe (III) protoporphyrin IX forms, which show the peak of absorption around 400–500 nm [134]. However, differences in endogenous porphyrin structures can modulate the lethality of PBM. For instance, the Prevotellaceae are black-pigmented bacteria accumulating protoporphyrin IX. However, P. intermedia integrates that with coproporphyrin III, whereas P. nigrescens with uroporphyrin III and heptacarboxyl porphyrin III. This could explain the difference in sensitivity or resistance to PBM observed in these genera.
It is important to note that the indirect effect of PBM should also be considered. For example, saliva plays a pivotal role in maintaining a healthy oral cavity and promotes the natural beneficial relationship between the oral microbiota and the host [135]. The reduced salivary secretion [136], low salivary pH, and altered salivary composition can change the oral cavity microbiota leading to dysbiosis associated with the risk of oral diseases [137]. Photobiomodulation has been employed to improve the functionality of the salivary glands acting on the salivary flow and increasing the salivary pH [138]. Moreover, as shown by Li et al. [139], “PBM therapy increased salivary levels of interleukin-1 receptor antagonist, interleukin-10, total antioxidant capacity, and catalase, and reduced the levels of tumor necrosis factor and interleukin, malondialdehyde, and 8-hydroxydeoxyguanosine”.
Additionally, Ailioaie and Litscher [140] discussed the potential role of PBM in the management of microbiota and the immune system and how the therapy can modulate their interconnection. Therefore, it was proposed that PBM could be beneficial to the normal microbiome recovery, stimulating the immune system [141].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031372
References
- Albini, A. Some remarks on the first law of photochemistry. Photochem. Photobiol. Sci. 2016, 15, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Pastore, D.; Greco, M.; Passarella, S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int. J. Radiat. Biol. 2000, 76, 863–870. [Google Scholar] [CrossRef]
- Karu, T.I. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 2010, 62, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Signore, A.; Ravera, S. Photobiomodulation and oxidative stress: 980 nm diode-laser light regulates mitochondria activity and reactive oxygen species production. Oxid. Med. Cell. Longev. 2021, 3, 6626286. [Google Scholar] [CrossRef]
- Ravera, S.; Ferrando, S.; Agas, D.; De Angelis, N.; Raffetto, M.; Sabbieti, M.G.; Signore, A.; Benedicenti, S.; Amaroli, A. 1064 nm Nd:YAG laser light affects transmembrane mitochondria respiratory chain complexes. J. Biophotonics 2019, 12, 201900101. [Google Scholar] [CrossRef]
- Swartz, T.E.; Corchnoy, S.B.; Christie, J.M.; Lewis, J.W.; Szundi, I.; Briggs, W.R.; Bogomolni, R.A. The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J. Biol. Chem. 2001, 276, 36493–36500. [Google Scholar] [CrossRef] [PubMed]
- Buravlev, E.A.; Zhidkova, T.V.; Vladimirov, Y.A.; Osipov, A.N. Effects of low-level laser therapy on mitochondrial respiration and nitrosyl complex content. Lasers Med. Sci. 2014, 29, 861–1866. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Borisov, S.M.; Saf, R.; Klimant, I. Strongly Phosphorescent Iridium(III) Porphyrins—New Oxygen Indicators with Tuneable Photophysical Properties and Functionalities. Eur. J. Inorg. Chem. 2011, 10, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1, 9. [Google Scholar] [CrossRef]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Applications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.-Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation of human adipose-derived stem cells using 810 nm and 980 nm lasers operates via different mechanisms of action. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Wu, M.; van der Steen, A.F. Photoacoustic imaging of human coronary atherosclerosis in two spectral bands. Photoacoustics 2013, 5, 12–20. [Google Scholar]
- Castellano-Pellicena, I.; Uzunbajakava, N.E.; Mignon, C.; Raafs, B.; Botchkarev, V.A.; Thornton, M.J. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg. Med. 2019, 51, 370–382. [Google Scholar] [CrossRef]
- Verbon, E.H.; Post, J.A.; Boonstra, J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene 2012, 511, 1–6. [Google Scholar] [CrossRef]
- Whitaker, M.; Patel, R. Calcium and cell cycle control. Development 1990, 108, 525–542. [Google Scholar] [CrossRef]
- Villalobo, A. Nitric oxide and cell proliferation. FEBS J. 2006, 273, 2329–2344. [Google Scholar] [CrossRef]
- Ravera, S.; Colombo, E.; Pasquale, C.; Benedicenti, S.; Solimei, L.; Signore, A.; Amaroli, A. Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. Int. J. Mol. Sci. 2021, 22, 4347. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef]
- Amaroli, A.; Colombo, E.; Zekiy, A.; Aicardi, S.; Benedicenti, S.; De Angelis, N. Interaction between Laser Light and Osteoblasts: Photobiomodulation as a Trend in the Management of Socket Bone Preservation—A Review. Biology 2020, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Agas, D.; Hanna, R.; Benedicenti, S.; De Angelis, N.; Sabbieti, M.G.; Amaroli, A. Photobiomodulation by Near-Infrared 980-nm Wavelengths Regulates Pre-Osteoblast Proliferation and Viability through the PI3K/Akt/Bcl-2 Pathway. Int. J. Mol. Sci. 2021, 22, 7586. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, C.; Utyuzh, A.; Mikhailova, M.V.; Colombo, E.; Amaroli, A. Recovery from Idiopathic Facial Paralysis (Bell’s Palsy) Using Photobiomodulation in Patients Non-Responsive to Standard Treatment: A Case Series Study. Photonics 2021, 8, 341. [Google Scholar] [CrossRef]
- Cassano, P.; Petrie, S.R.; Hamblin, M.R.; Henderson, T.A.; Iosifescu, D.V. Review of transcranial photobiomodulation for major depressive disorder: Targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 2016, 3, 031404. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Benedicenti, S.; Amaroli, A.; Sălăgean, T.; Pop, I.D.; Todea, D.; Bordea, I.R. Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future. Cancers 2020, 12, 1949. [Google Scholar] [CrossRef]
- Zadik, Y.; Arany, P.R.; Fregnani, E.R.; Bossi, P.; Antunes, H.S.; Bensadoun, R.J.; Gueiros, L.A.; Majorana, A.; Nair, R.G.; Ranna, V. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3969–3983. [Google Scholar]
- Ravera, S.; Bertola, N.; Pasquale, C.; Bruno, S.; Benedicenti, S.; Ferrando, S.; Zekiy, A.; Arany, P.; Amaroli, A. 808-nm Photobiomodulation Affects the Viability of a Head and Neck Squamous Carcinoma Cellular Model, Acting on Energy Metabolism and Oxidative Stress Production. Biomedicines 2021, 9, 1717. [Google Scholar] [CrossRef]
- De Pauli Paglioni, M.; Araújo, A.L.D.; Arboleda, L.P.A.; Palmier, N.R.; Fonsêca, J.M.; Gomes-Silva, W.; Madrid-Troconis, C.C.; Silveira, F.M.; Martins, M.D.; Faria, K.M. Tumor safety and side effects of photobiomodulation therapy used for prevention and management of cancer treatment toxicities. A systematic review. Oral Oncol. 2019, 93, 21–28. [Google Scholar] [CrossRef]
- Bensadoun, R.J.; Epstein, J.B.; Nair, R.G.; Barasch, A.; Raber-Durlacher, J.E.; Migliorati, C.; Genot-Klastersky, M.T.; Treister, N.; Arany, P.; Lodewijckx, J. Safety and efficacy of photobiomodulation therapy in oncology: A systematic review. Cancer Med. 2020, 9, 8279–8300. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Mouridsen, H.T.; Bergmann, O.J.; Reibel, J.; Breunner, N.; Nauntofte, B. Oral mucosal lesions, microbial changes, and taste disturbances induced by adjuvant chemotherapy in breast cancer patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Bicknell, B.; Johnstone, D.M.; Gordon, L.C.; Kiat, H.; Hamblin, M.R. “Photobiomics”: Can Light, Including Photobiomodulation, Alter the Microbiome? Photobiomodul. Photomed. Laser Surg. 2019, 37, 681–693. [Google Scholar] [CrossRef]
- Pflughoeft, K.J.; Versalovic, J. Human microbiome in health and disease. Annu. Rev. Pathol. 2012, 7, 99–122. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G. Eukaryotic-microbiota cross talk: Potential mechanisms for health benefits of prebiotics and probiotics. Annu. Rev. Nutr. 2008, 28, 215–231. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Di Spirito, F.; La Rocca, M.; De Bernardo, M.; Rosa, N.; Sbordone, C.; Sbordone, L. Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study. Dent. J. 2021, 9, 1. [Google Scholar] [CrossRef]
- Di Spirito, F.; Toti, P.; Pilone, V.; Carinci, F.; Lauritano, D.; Sbordone, L. The Association between Periodontitis and Human Colorectal Cancer: Genetic and Pathogenic Linkage. Life 2020, 10, 211. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Chatelain, S.; Derchi, G.; Di Spirito, F.; Martuscelli, R.; Porzio, M.; Sbordone, L. Antibiotic’s effectiveness after erupted tooth extractions: A retrospective study. Oral Dis. 2020, 26, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Bunce, J.; Hellyer, P. Antibiotic resistance and antibiotic prescribing by dentists in England 2007–2016. Br. Dent. J. 2018, 225, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Lighthart, B. Solar radiation is shown to select for pigmented bacteria in the ambient outdoor atmosphere. Photochem. Photobiol. 1997, 65, 103–106. [Google Scholar] [CrossRef]
- Trushin, M.V. Studies on distant regulation of bacterial growth and light emission. Microbiology 2003, 149, 363–368. [Google Scholar] [CrossRef]
- Lubart, R.; Lipovski, A.; Nitzan, Y.; Friedmann, H. A possible mechanism for the bactericidal effect of visible light. Laser Ther. 2011, 20, 17–22. [Google Scholar] [CrossRef]
- Bordea, I.R.; Hanna, R.; Chiniforush, N.; Grădinaru, E.; Câmpian, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation of the outcome of various laser therapy applications in root canal disinfection: A systematic review. Photodiagn. Photodyn. Ther. 2020, 29, 101611. [Google Scholar] [CrossRef]
- Bicknell, B.; Liebert, A.; Johnstone, D.; Kiat, H. Photobiomodulation of the microbiome: Implications for metabolic and inflammatory diseases. Lasers Med. Sci. 2019, 34, 317–327. [Google Scholar] [CrossRef]
This entry is offline, you can click here to edit this entry!
