Microscopic prokaryotic and eukaryotic algae (microalgae), which can be effectively grown in mass cultures, are gaining increasing interest in cosmetics. Up to now, the main attention was on aquatic algae, while species from aeroterrestrial and extreme environments remained underestimated. In these habitats, algae accumulate high amounts of some chemical substances or develop specific compounds, which cause them to thrive in inimical conditions. Among such biologically active molecules is a large family of lipids, which are significant constituents in living organisms and valuable ingredients in cosmetic formulations. Therefore, natural sources of lipids are increasingly in demand in the modern cosmetic industry and its innovative technologies. Among novelties in skin care products is the use of lipid nanoparticles as carriers of dermatologically active ingredients, which enhance their penetration and release in the skin strata.
- carotenoids
- Cyanoprokaryota
- fatty acids
- morphological type
- PUFA
- Ochrophyta
- Rhodophyta
- Chlorophyta
- Streptophyta
- sterols
1. Introduction
2. Current Insights
| Taxonomic Group/Alga | Ecological Group | Investigated Lipid Classes with Examples of Detected Lipids | References |
|---|---|---|---|
| CYANOPROKARYOTA | |||
| Anabaena cylindrica | AET | FA (PUFA—linoleic and linolenic acids, SAFA—palmitic acid and MUFA); GL; ST | [34] |
| Anabaena cylindrica 1403-2 | AET | GL (MGD, DGD, SQD and PSD) | [35] |
| Anabaena vaginicola | AET | PR (lycopene, lutein, beta-carotene, zeaxanthin) | [36] |
| Calothrix sp. | AET | ST | [34][37][38][39][40] |
| Desmonostoc muscorum | AET | FA (PUFA—hexadecadienoic and linoleic acids, SAFA—palmitic acid, MUFA—oleic and palmitoleic acids); GL; ST | [34][37][38][39][40] |
| Drouetiella lurida | AET—soil, subaerial | ST (seven unsaturated ST) | [41] |
| Microcoleus autumnalis | AET—soil, subaerial | ST (cholesterol, β-sitosterol and stigmasterol with squalene as a precursor; ergosterol) | [40] |
| Nostoc calcarea | AET—soil, subaerial | PR (lycopene, lutein, beta-carotene, zeaxanthin) | [36] |
| Nostoc calcicola B 1459-2 | AET—soil, subaerial | FA (PUFA—linolenic acid, SAFA, MUFA), GL—MGD, DGD, SQD and PSD | [35] |
| Nostoc carneum | AET—soil, subaerial | ST | [34][37][38][39][40] |
| “Nostoc canina” | AET (symbiont?) |
FA (PUFA— linoleic acid, SAFA -palmitic acid, MUFA—palmitoleic and oleic acids); GL; ST (cholesterol and lanosterol) | [34] |
| Nostoc commune | AET | ST | [34][37][38][39][40] |
| Nostoc commune var. sphaeroides | AET | ST (campesterol, sitosterol and clionasterol) | [42][39][43] |
| Nostoc punctiforme PCC73102 | AET | FA (FAEs—oxylipins), PR (genes for ASX and canthaxanthin) | [44][45] |
| Nostoc sp. PCC7120 | AET | FA (FAEs—oxylipins) | [46] |
| Oscillatoria chalybea B1459-2 | AET | GL (MGD, DGD, TGD, SQD and PSD) | |
| Oscillatoria sp. PBGA3 | AET—soil | FA (FtAs) | [47] |
| Scytonema sp. | AET | ST (cholest-5-en-3β-ol (18.9 %), 3β-methoxycholest-5-ene (16.2 %) and 3β-acetoxycholest-5-ene (11.2 %), ergosta-5,7,22,24(28)-tetraen-3β-ol) | [48] |
| Tolypothrix tenuis B1482-3 | AET | GL (MGD, DGD, TGD, SQD and PSD) | [35] |
| Tolypothrix sp. PBGA1 | AET | FA (FtAs) | [47] |
| Tolypothrix sp. PBGA2 | AET | FA (FtAs) | [47] |
| RHODOPHYTA | |||
| Cyanidium caldarium | EXT—thermal springs | GL; ST (ergosta-5,7,22,24(28)-tetraen-3β-ol) | [49] |
| Cyanidioschyzon merolae | EXT—thermal springs | PK | [50] |
| Galdieria sulfuraria (>47 strains) | EXT—thermal springs/AET—cryptoendolith | FA (PUFA—linoleic and linolenic acid, SAFA—palmitic acid, MUFA—oleic and palmitoleic acids); GL (MGD, DGD and SQD; PG, PC, PE, PI, PS and phosphatidate); GP; ST (ergosta-5,7,22,24(28)-tetraen-3β-ol and ergosterol); PR (ß-carotene, lutein); PK | [51][52][53][49][50] |
| Galdieria sulfuraria/Galdieria sp. | EXT—acidic but non-thermophilic | FA (PUFA—linoleic and linolenic acids, SAFA—palmitic, myristic and stearic acids, MUFA—oleic and palmitoleic acids) | [51][52] |
| Galdieria sp. USB-GBX-832 | EXT—thermal springs | FA (PUFA—linoleic acid, AA and EPA; SAFA—palmitic and stearic acid, MUFA—oleic acid) | [54] |
| Pophyridium purpureum | AET-soil | FA (PUFA—AA and EPA); GL | [55] |
| OCHROPHYTA | |||
| Eustigmatophyceae | |||
| Monodopsis subterraneus | AET—soil | FA (PUFA—EPA), GL (DGD) | [56][57][58][59][60][61] |
| Monodus guttula | AET | PR (tocopherols) | [2] |
| Monodus sp. | AET | PR (carotenoids—ASX, beta-carotene and lutein) | [62] |
| Vischeria/Eustigmatos | AET—soil, subaerial | PR (total carotenoids; ASX, beta-carotene, lutein and canthaxanthin) | [62] |
| Tribophyceae (=Xanthophyceae) | |||
| Botrydiopsis interdecens | AET | PR (tocopherols) | [2] |
| Heterococcus sp. | AET | PR (tocopherols) | [2] |
| Xanthonema sp. | AET | PR (tocopherols) | [2] |
| CHLOROPHYTA | |||
| Acutodesmus dissociatus TGA1 | AET—soil | FA (SAFA—palmitic acid and MUFA—oleic acid) | [47] |
| Auxenochlorella protothecoides | AET/EXT—acidic | PR (carotenoids—lutein) | [63][64][65][66][67][68][69] |
| Auxenochlorella pyrenoidosa | AET | PR (carotenoids—ASX, zeaxanthin, canthaxanthin, lutein) | [70][71][72][73] |
| Bracteacoccus sp. | AET | PR (tocopherols) | [2] |
| Chlamydocapsa sp. | EXT—snow | PR (canthaxanthin, tocopherols) | [2][74] |
| Chlamydomonas nivalis | EXT—snow | PR (ASX, canthaxanthin) | [74][75][76] |
| Chlamydomonas reinhardtii | AET | FA (hydrocarbons—C17 alkene n-heptadecene), GL (betaine lipids—DGTS); GP; ST (ergosterol); PK | [77][78][79][50][80][81] |
| Chlainomonas sp. | EXT—snow | PR (ASX) | [82] |
| Chlorella sorokiniana | AET | ST (ergosterol) | [79] |
| Chlorella variabilis | AET | PK | [50][80][81] |
| Chlorella variabilis NC64A | AET (symbiotic) | ST (ergosterol) | [79] |
| Chlorella vulgaris | AET | FA (free FtAs, FAEs—lactones; hydrocarbons—NC64A eptadecane pentadecane, as well as 7- and 8-heptadecene); GL; ST (ergosterol, 7-dehydroporiferasterol, ergosterol peroxide, 7-dehydroporiferasterol per-oxide and 7-oxocholesterol); PR (carotenoids—ASX, zeaxanthin, canthaxanthin and lutein), PK | [21][24][83][84][77][85][70][71][72][73] |
| Chlorella sp. PGA2 | AET—soil | FA (SAFA, MUFA) | [47] |
| Chlorella sp. TGA2 | AET—soil | FA (SAFA- palmitic acid, MUFA—oleic acid) | [47] |
| Chlorella sp. TGA4 | AET—soil | FA (SAFA, MUFA) | [47] |
| Chlorococcum sp. (1) | AET | PR (carotenoids—ASX (in a free form and as esters), adonixanthin (in a free form and as esters), lutein, canthaxanthin and β-carotene) | [86][87][88][89][90][91][92] |
| Chlorococcum sp. MA-1 | AET | PR (total carotenoids; ASX, lutein, canthaxanthin and ß-carotene) | [88] |
| Chlorococcum spp. | EXT—snow | PR (ß-carotene, lutein and canthaxanthin) | [51][93][74][75][76][94][95] |
| Chloroidium ellipsoideum | AET | PR (carotenoids—zeaxanthin) | [96] |
| Chloromonas alpina | EXT—snow | FA (PUFA, SAFA, MUFA), PR (ASX) | [51][93][97][98][75][76][94][95] |
| Chloromonas hindakii | EXT—snow | FA (PUFA—α-linolenic, stereadonic and hexadecatetraenoic acids, SAFA—palmitic acid and MUFA—oleic acid); GP; PR (ASX) | [51][93][97][98][75][76][94][95] |
| Chloromonas nivalis | EXT—snow | FA (PUFA—hexadecatetraenoic, SAFA and MUFA); PR (ASX, canthaxanthin) | [51][93][97][98][74][75][76][94][95] |
| Chloromonas nivalis subsp. tatrae | EXT—snow | FA (PUFA, SAFA and MUFA); PR (ASX) | [98] |
| Chloromonas polyptera | EXT—snow | FA (PUFA, SAFA and MUFA), PR (ASX) | [51][93][97][98][75][76][94][95] |
| Chloromonas remiasii CCCryo 005–99 | EXT—snow | FA (PUFA-hexadecatetraenoic acid, SAFA and MUFA), PR | [99][51][93][97][98][75][76][94][95] |
| Chloromonas spp. | EXT—snow | FA (PUFA, SAFA—palmitic acid and MUFA—oleic acid), PR | [93][97][98][75][76][94][95] |
| Chromochloris zofingiensis | AET | PR (carotenoids—ASX, canthaxanthin, zeaxanthin, lutein and β-carotene) | [70][86][87][100][101][102][103][104][105][106][107][108][109] |
| Coccomyxa acidophila | EXT—acidic | PR (carotenoids—ß-carotene and lutein) | [110] |
| Coccomyxa subellipsoidea | AET | ST (phytosterols) | [79] |
| Coccomyxa subellipsoidea C-169 | AET | PK | [50][80][81] |
| Coccomyxa sp. | AET | PR (tocopherols) | [2] |
| Coelastrella oocystiformis | AET | PR (carotenoids—ASX esters and canthaxanthin) | [101][109] |
| Coelastrella striolata var. multistriolata | AET—subaerial, soils | FA (PUFA—linoleic acid, SAFA—palmitic acid and MUFA—oleic acid); PR (carotenoids—canthaxanthin, ASX and ß-carotene) | [111][112] |
| Dunaliella acidophila | EXT-acidic | FA (PUFA—linolenic, γ-linolenic and linoleic acids; SAFA; MUFA—oleic and elaidic acids; FAEs—lactones, methyl (12R)-hydroxyoctadeca-9Z,13E,15Z-trienoate, methyl (9S)-hydroxyoctadeca-10E, 12Z,15Z-trienoate and methyl ricinoleate; triacylglycerols—trilinolenin, triolein, trielaidin and tristearin); ST (β-sitosterol, isofucosterol, 24-methylenlophenol, (24S)-methyllophenol and two unidentified sterols, acylsterols and phytol); PR (lycopene, alpha-, beta and gamma-carotene) | [113][77] |
| Edaphochlamys debaryana | AET—soil | FA (FAEs—oxylipins) | [114] |
| Hindakia tetrachotoma PGA1 | AET—soil | FA (SAFA—palmitic acid and MUFA—oleic acid) | [47] |
| Monoraphidum sp. | EXT—ice | FA (PUFA) | [31] |
| Muriella terrestris | AET | PR (tocopherols) | [2] |
| Muriellopsis sp. | AET | PR (carotenoids—lutein) | [115][116][117][118][119] |
| Neochloris wimmeri | AET | PR (carotenoids—ASX esters and canthaxanthin) | [101][109] |
| Parietochloris alveolaris | AET-—oil, symbiont | FA (PUFA—EPA, AA and its precursor dihomo-γ-linolenic acid) | [120][121] |
| Parietochloris alveolaris K-1 | AET | FA (PUFA—α-linolenic acid and EPA) | [122][123] |
| Protosiphon botryoides | AET—soil | PR (carotenoids—ASX esters and canthaxanthin) | [101][109] |
| Pseudochoricystis ellipsoidea MBIC11204 | EXT—thermal springs | FA (FtAs and FAEs—hydrocarbons and triacylglycerols) | [124] |
| Raphidonema sempervirens | EXT—snow | FA (PUFA, SAFA and MUFA); PR (ß-carotene, ASX, lutein and tocopherols) | [2][93][97][98][74] |
| Sanguina aurantia | EXT—snow | PR (ASX) | [125] |
| Sanguina nivalis | EXT—snow | PR (ASX) | [125] |
| Scenedesmus vacuolatus | AET | PR (carotenoids—ASX esters and canthaxanthin) | [101][109] |
| Scenedesmus spp. | AET | PR (total carotenoids, ASX and lutein) | [112][126][127] |
| Stichococcus bacillaris | AET | PR (tocopherols) | [2] |
| Tetracystis sp. | AET/EXT—cryotolerant | PR (canthaxanthin) | [128] |
| Tetradesmus obliquus | AET | FA (PUFA—linolenic, linoleic and linolelaidic acids and SAFA—oleic acid); PR (carotenoids—ASX and lutein) | [21][25][112][129][130][131][132][133][126][127] |
| Tetradesmus obliquus (strain Scenedesmus obliquus SNW-N) | AET | PR (lutein) | [126] |
| Tetradesmus obliquus (strain Scenedesmus obliquus FSP-3) | AET | PR (lutein) | [134] |
| Ulothrix zonata | EXT—ice | FA (PUFA) | [111] |
| “Unidentified Chlamydomonadaceae” | EXT—snow | FA (PUFA, SAFA and MUFA); PR (ASX) | [47] |
| Unidentified “Chlamydomonadales species” TGA3 | AET—soil, thermotolerant | FA (SAFA and MUFA) | [47] |
| Unidentified “Chlamydomonadales species” TGA5 | AET—soil | FA (SAFA and MUFA) | [47] |
| STREPTOPHYTA | |||
| Klebsormidium flaccidum | AET | PK | [50] |
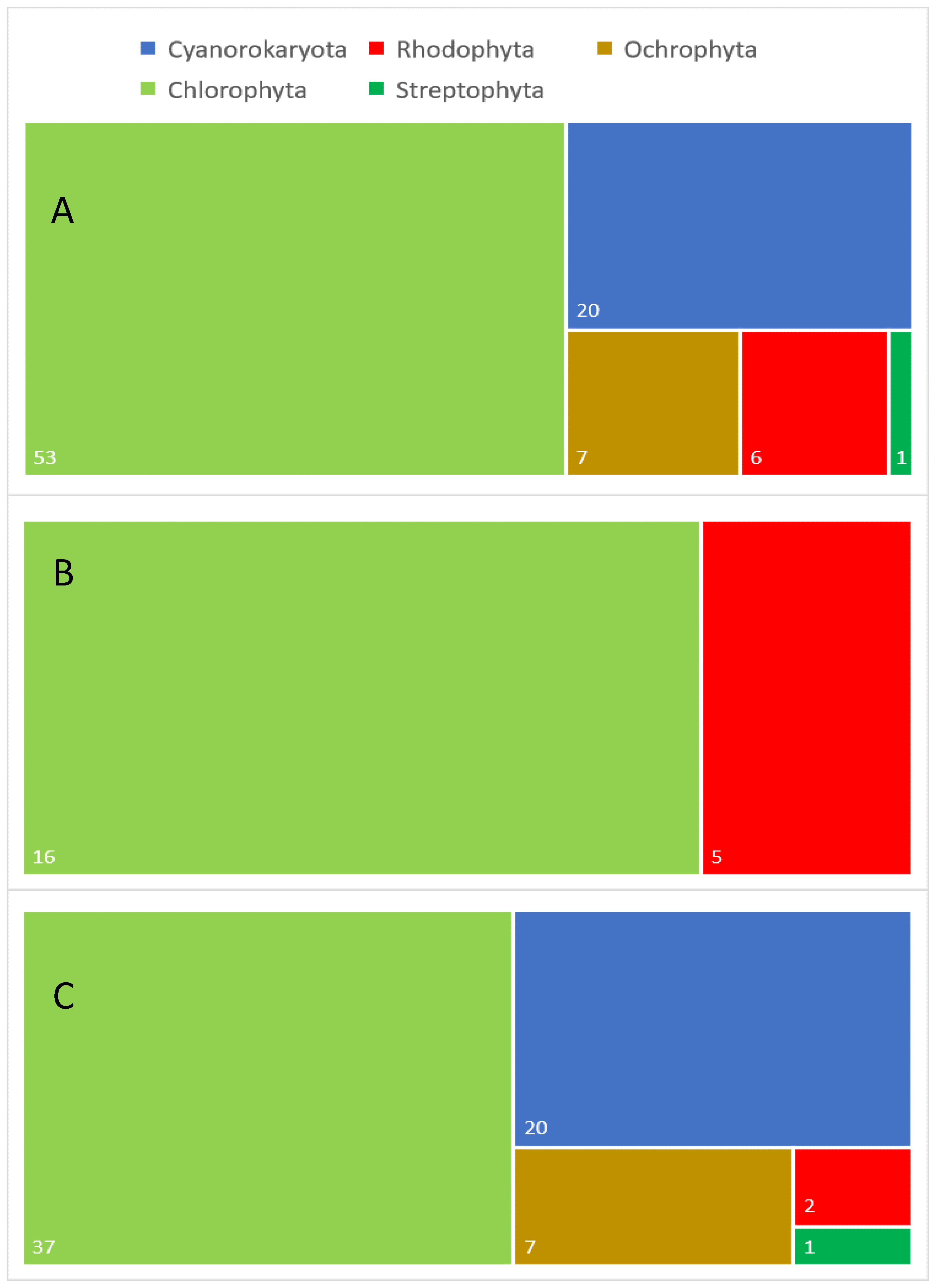 Figure 1. Taxonomic diversity of aeroterrestrial and extremophilic microalgae analyzed for different lipids: (A) general taxonomic diversity of the analyzed algal species, (B) taxonomic diversity of the examined extremophilic species and (C) taxonomic diversity of the investigated aeroterrestrial species. Numbers in white indicate the exact number in each category.
Figure 1. Taxonomic diversity of aeroterrestrial and extremophilic microalgae analyzed for different lipids: (A) general taxonomic diversity of the analyzed algal species, (B) taxonomic diversity of the examined extremophilic species and (C) taxonomic diversity of the investigated aeroterrestrial species. Numbers in white indicate the exact number in each category.3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cosmetics9010011
References
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487.
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G. Enigmatic microalgae from aeroterrestrial and extreme habitats in cosmetics: The potential of the untapped natural sources. Cosmetics 2020, 7, 27.
- Sun, Z.; Li, T.; Zhou, Z.; Jiang, Y. Microalgae as a source of lutein: Chemistry, biosynthesis, and carotenogenesis. In Microalgae Biotechnology. Advances in Biochemical Engineering/Biotechnology; Posten, C., Feng, C.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 153, pp. 37–58.
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 7, 1269–1278.
- Jahan, A.; Ahmad, I.Z.; Fatima, N.; Ansari, V.A.; Akhtar, J. Algal bioactive compounds in the cosmeceutical industry: A review. Phycologia 2017, 56, 410–422.
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362.
- Basily, H.S.; Nassar, M.M.; Diwani, G.I.; Abo El-Enin, S.A. Exploration of using the algal bioactive compounds for cosmeceuticals and pharmaceutical applications. Egypt. Pharm. J. 2018, 17, 109–120. Available online: http://www.epj.eg.net/text.asp?2018/17/2/109/240673 (accessed on 25 November 2021).
- Rastogi, R.P.; Sinha, R.P. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 2009, 27, 521–539.
- Bonnet, C. Lipids, a natural raw material at the heart of cosmetics innovation. OCL 2018, 25, D501.
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from microalgae for cosmetic applications. Cosmetics 2021, 8, 52.
- Knox, S.; O’Boyle, N.M. Skin lipids in health and disease: A review. Chem. Phys. Lipids 2021, 236, 105055.
- Kabri, T.; Arab-Tehrany, E.; Belhaj, N.; Linder, M. Physico-chemical characterization of nano-emulsions in cosmetic matrix enriched on omega-3. J. Nanobiotechnol. 2011, 9, 41.
- Sarkar, R.D.; Singh, H.B.; Kalita, M.C. Enhanced lipid accumulation in microalgae through nanoparticle-mediated approach, for biodiesel production: A mini-review. Heliyon 2021, 7, e08057.
- Khater, D.; Nsairat, H.; Odeh, F.; Saleh, M.; Jaber, A.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Design, preparation, and characterization of effective dermal and transdermal lipid nanoparticles: A review. Cosmetics 2021, 8, 39.
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95.
- Khezri, K.; Saeedi, M.; Dizaj, S.M. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505.
- Alvarez, A.M.R.; Rodríguez, M.L.G. Lipids in pharmaceutical and cosmetic preparations. Grasas Aceites 2000, 51, 74–96. Available online: https://grasasyaceites.revistas.csic.es/index.php/grasasyaceites/article/view/409 (accessed on 3 November 2021).
- Zielinska, A.; Nowak, I. Fatty acids in vegetable oils and their importance in cosmetic industry. CHEMIK Nauka Tech. Rynek 2014, 68, 103–110.
- Ahmad, J. Lipid nanoparticles based cosmetics with potential application in alleviating skin disorders. Cosmetics 2021, 8, 84.
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 2020, 8, 434.
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232.
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96.
- McKie-Krisberg, Z.M.; Laurens, L.M.L.; Huang, A.; Polle, J.E.W. Comparative energetics of carbon storage molecules in green algae. Algal Res. 2018, 31, 326–333.
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278.
- Pignolet, O.; Jubeau, S.; Vaca-Garcia, C.; Philippe, M. Highly valuable microalgae: Biochemical and topological aspects. J. Ind. Microbiol. Biotechnol. 2013, 40, 781–796.
- Wilkie, A.C.; Edmundson, S.J.; Duncan, J.G. Indigenous algae for local bioresource production: Phycoprospecting. Energy Sustain. Dev. 2011, 15, 365–371.
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G.; Radkova, M.; Atanassov, I.; Atanassova, R.; Borisova, C.; Draganova, P.; Stoykova, P. Review on the biotechnological and nanotechnological potential of the streptophyte genus Klebsormidium with pilot data on its phycoprospecting and polyphasic identification in Bulgaria. Biotechnol. Biotechnol. Equip. 2019, 33, 559–578.
- Aratboni, H.A.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Fact. 2019, 18, 178.
- Slocombe, S.P.; Zhang, Q.Y.; Ross, M.; Anderson, A.; Thomas, N.J.; Lapresa, Á.; Rad-Menéndez, C.; Campbell, C.N.; Black, K.D.; Stanley, M.S.; et al. Unlocking nature’s treasure-chest: Screening for oleaginous algae. Sci. Rep. 2015, 5, 9844.
- Richter, C.K.; Skulas-Ray, A.C.; Kris-Etherton, P.M. Recommended intake of fish and fish oils worldwide. In Fish and Fish Oil in Health and Disease Prevention; Raatz, S.K., Bibus, D.M., Eds.; Elsevier: London, UK, 2016; pp. 27–48.
- Řezanka, T.; Nedbalová, L.; Lukavský, J.; Střížek, A.; Sigler, K. Pilot cultivation of the green alga Monoraphidium sp. producing a high content of polyunsaturated fatty acids in a low-temperature environment. Algal Res. 2017, 22, 160–165.
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861.
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14.
- Sallal, A.K.; Nimer, N.A.; Radwan, S.S. Lipid and fatty acid composition of freshwater cyanobacteria. Microbiology 1990, 136, 2043–2048.
- Zepke, H.D.; Heinz, E.; Radunz, A.; Linscheid, M.; Pesch, R. Combination and positional distribution of fatty acids in lipids from blue-green algae. Arch. Microbiol. 1978, 119, 157–162.
- Hashtroudi, M.S.; Shariatmadari, Z.; Riahi, H.; Ghassempour, A. Analysis of Anabaena vaginicola and Nostoc calcicola from Northern Iran, as rich sources of major carotenoids. Food Chem. 2013, 136, 1148–1153.
- Fagundes, M.B.; Wagner, R. Sterols biosynthesis in algae. In Bioactive Compounds. Biosynthesis, Characterization and Applications; Zepka, L.Q., Ed.; IntechOpen: London, UK, 2021.
- Konlhase, M.; Pohl, P. Saturated and unsaturated sterols of nitrogen-fixing blue-green algae (cyanobacteria). Phytochemistry 1988, 27, 1735–1740.
- Paoletti, C.; Pushparaj, B.; Florenzano, G.; Capella, P.; Lercker, G. Unsaponifiable matter of green and blue-green algal lipids as a factor of biochemical differentiation of their biomasses: II. Terpenic alcohol and sterol fractions. Lipids 1976, 11, 266–271.
- Fagundes, M.B.; Falk, R.B.; Facchi, M.M.X.; Vendruscolo, R.G.; Maroneze, M.M.; Zepka, L.Q.; Wagner, R. Insights in cyanobacteria lipidomics: A sterols characterization from Phormidium autumnale biomass in heterotrophic cultivation. Food Res. Int. 2019, 119, 777–784.
- De Souza, N.J.; Nes, W.R. Sterols: Isolation from a bluegreen alga. Science 1968, 162, 363.
- Luo, X.; Su, P.; Zhang, W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar. Drugs 2015, 13, 4231–4254.
- Rasmussen, H.E.; Blobaum, K.R.; Park, Y.-K.; Ehlers, S.J.; Lu, F.; Lee, J.-Y. Lipid extract of Nostoc commune var. sphaeroides Kützing, a blue-green alga, inhibits the activation of sterol regulatory element binding proteins in HepG2 Cells. J. Nutr. 2008, 138, 476–481.
- Lang, I.; Feussner, I. Oxylipin formation in Nostoc punctiforme (PCC73102). Phytochemistry 2007, 68, 1120–1127.
- Steiger, S.; Sandmann, G. Cloning of two carotenoid ketolase genes from Nostoc punctiforme for the heterologous production of canthaxanthin and astaxanthin. Biotechnol. Lett. 2004, 26, 813–817.
- Lang, I.; Göbel, C.; Porzel, A.; Heilmann, I.; Feussner, I. A lipoxygenase with linoleate diol synthase activity from Nostoc sp. PCC 7120. Biochem. J. 2008, 410, 347–357.
- Thangavel, K.; Krishnan, P.R.; Nagaiah, S.; Kuppusamy, S.; Chinnasamy, S.; Rajadorai, J.S.; Olaganathan, G.N.; Dananjeyan, B. Growth and metabolic characteristics of oleaginous microalgal isolates from Nilgiri biosphere Reserve of India. BMC Microbiol. 2018, 18, 1.
- Řezanka, T.; Dembitsky, V.M.; Go, J.V. Sterol compositions of the filamentous nitrogen-fixing terrestrial cyanobacterium Scytonema sp. Folia Microbiol. 2003, 48, 357–360.
- Seckbach, J.; Ikan, R.; Ringelberg, D.; White, D. Sterols and phylogeny of the acidophilic hot springs algae Cyanidium caldarium and Galdieria sulphuraria. Phytochemistry 1993, 34, 1345–1349.
- Shelest, E.; Heimerl, N.; Fichtner, M.; Sasso, S. Multimodular type I polyketide synthases in algae evolve by module duplications and displacement of AT domains in trans. BMC Genomics 2015, 16, 1015.
- Procházková, L.; Remias, D.; Řezanka, T.; Nedbalová, L. Ecophysiology of Chloromonas hindakii sp. nov. (Chlorophyceae), causing orange snow blooms at different light conditions. Microorganisms 2019, 7, 434.
- Graziani, G.; Schiavo, S.; Nicolai, M.A.; Buono, S.; Fogliano, V.; Pinto, G.; Pollio, A. Microalgae as human food: Chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct. 2013, 4, 144–152.
- Vítová, M.; Goecke, F.; Sigler, K.; Řezanka, T. Lipidomic analysis of the extremophilic red alga Galdieria sulphuraria in response to changes in pH. Algal Res. 2016, 13, 218–226.
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of polyunsaturated fatty acids and lipids from autotrophic, mixotrophic and heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791.
- Guil-Guerrero, J.; Belarbi, E.H.; Rebolloso-Fuentes, M. Eicosapentaenoic and arachidonic acids purification from the red microalga Porphyridium cruentum. Bioseparation 2000, 9, 299–306.
- Iwamoto, H.; Sato, S. Production of EPA by freshwater unicellular algae. J. Am. Oil. Chem. Soc. 1968, 71, 434.
- Cohen, Z. Production potential of eicosapentaenoic acid by Monodus subterraneus. J. Am. Oil Chem. Soc. 1994, 71, 941–945.
- Khozin-Goldberg, I.; Didi-Cohen, S.; Cohen, Z. Biosynthesis of eicosapentaenoic acid (EPA) in the freshwater eustigmatophyte Monodus subterraneus (Eustigmatophyceae). J. Phycol. 2002, 38, 745–756.
- Lu, C.; Rao, K.; Hall, D.; Vonshak, A. Production of eicosapentaenoic acid (EPA) in Monodus subterraneus grown in a helical tubular photobioreactor as affected by cell density and light intensity. J. Appl. Phycol. 2001, 13, 517–522.
- Vazhappilly, R.; Chen, F. Eicosapentaenoic acid and docosahexaenoic acid production potential of microalgae and their heterotrophic growth. J. Am. Oil Chem. Soc. 1998, 75, 393–397.
- Khozin-Goldberg, I.; Cohen, Z. The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 2006, 67, 696–701.
- Stoyneva-Gärtner, M.; Stoykova, P.; Uzunov, B.; Dincheva, I.; Atanassov, I.; Draganova, P.; Borisova, C.; Gärtner, G. Carotenoids in five aeroterrestrial strains from Vischeria/Eustigmatos group: Updating the pigment patterns of Eustigmatophyceae. Biotechnol. Biotechnol. Equip. 2019, 33, 250–267.
- Huss, V.A.; Ciniglia, C.; Cennamo, P.; Cozzolino, S.; Pinto, G.; Pollio, A. Phylogenetic relationships and taxonomic position of Chlorella-like isolates from low pH environments (pH < 3.0). BMC Evol. Biol. 2002, 2, 13.
- Darienko, T.; Pröschold, T. Genetic variability and taxonomic revision of the genus Auxenochlorella (Shihira et Krauss) Kalina et Puncocharova (Trebouxiophyceae, Chlorophyta). J. Phycol. 2015, 51, 394–400.
- Gao, C.; Wang, Y.; Shen, Y.; Yan, D.; He, X.; Dai, J.; Wu, Q. Oil accumulation mechanisms of the oleaginous microalga Chlorella protothecoides revealed through its genome, transcriptomes, and proteomes. BMC Genomics 2014, 15, 582.
- Shi, X.-M.; Liu, H.-J.; Zhang, X.-W.; Chen, F. Production of biomass and lutein by Chlorella protothecoides at various glucose concentrations in heterotrophic cultures. Process Biochem. 1999, 34, 341–347.
- Shi, X.M.; Chen, F. Production and rapid extraction of lutein and the other lipid-soluble pigments from Chlorella protothecoides grown under heterotrophic and mixotrophic conditions. Nahrung 1999, 43, 109–113.
- Shi, X.-M.; Chen, F.; Yuan, J.P.; Chen, H. Heterotrophic production of lutein by selected Chlorella strains. J. Appl. Phycol. 1997, 9, 445–450.
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995.
- Gupta, A.K.; Seth, K.; Maheshwari, K.; Baroliya, P.K.; Meena, M.; Kumar, A.; Vinayak, V.; Harish. Biosynthesis and extraction of high-value carotenoid from algae. Front. Biosci. Landmark 2021, 26, 171–190.
- Bhosale, P.; Bernstein, P.S. Microbial xanthophylls. Appl. Microbiol. Biotechnol. 2005, 68, 445–455.
- Theriault, R.J. Heterotrophic growth and production of xanthophylls by Chlorella pyrenoidosa. Appl. Microbiol. 1965, 13, 402–416.
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.-G.; Lee, D.-U.; Pan, C.-H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797.
- Leya, T.; Rahn, A.; Lütz, C.; Remias, D. Response of arctic snow and permafrost algae to high light and nitrogen stress by changes in pigment composition and applied aspects for biotechnology. FEMS Microbiol. Ecol. 2009, 67, 432–443.
- Remias, D.; Lütz-Meindl, U.; Lütz, C. Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis. Eur. J. Phycol. 2005, 40, 259–268.
- Remias, D.; Lütz, C. Characterisation of esterified secondary carotenoids and of their isomers in green algae: A HPLC approach. Algol. Stud. 2005, 124, 85–94.
- Sorigué, D.; Légeret, B.; Cuiné, S.; Morales, P.; Mirabella, B.; Guédeney, G.; Li-Beisson, Y.; Jetter, R.; Peltier, G.; Beisson, F. Microalgae synthesize hydrocarbons from long-chain fatty acids via a light-dependent pathway. Plant Physiol. 2016, 171, 2393–2405.
- Giroud, C.; Gerber, A.; Eichenberger, W. Lipids of Chlamydomonas reinhardtii. Analysis of molecular species and intracellular site(s) of biosynthesis. Plant Cell Physiol. 1988, 29, 587–595.
- Voshall, A.; Christie, N.T.M.; Rose, S.L.; Khasin, M.; Van Etten, J.L.; Markham, J.E.; Riekhof, W.R.; Nickerson, K.W. Sterol Biosynthesis in four green algae: A bioinformatic analysis of the ergosterol versus phytosterol decision point. J. Phycol. 2021, 57, 1199–2111.
- Sasso, S.; Pohnert, G.; Lohr, M.; Mittag, M.; Hertweck, C. Microalgae in the post-genomic era: A blooming reservoir for new natural products. FEMS Microbiol. Rev. 2013, 36, 761–785.
- John, U.; Beszteri, B.; Derelle, E.; Van de Peer, Y.; Read, B.; Moreau, H.; Cembella, A. Novel insights into evolution of protistan polyketide synthases through phylogenomic analysis. Protist 2008, 159, 21–30.
- Remias, D.; Pichrtová, M.; Pangratz, M.; Lütz, C.; Holzinger, A. Ecophysiology, secondary pigments and ultrastructure of Chlainomonas sp. (Chlorophyta) from the European Alps compared with Chlamydomonas nivalis forming red snow. FEMS Micorbiol. Ecol. 2016, 92, fiw030.
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994.
- Lee, R.E. Phycology, 4th ed.; Cambridge University Press: New York, NY, USA, 2008.
- Yasukawa, K.; Akihisa, T.; Kanno, H.; Kaminaga, T.; Izumida, M.; Sakoh, T.; Tamura, T.; Takido, M. Inhibitory effects of sterols isolated from Chlorella vulgaris on 12-O-tetradecanoylphorbol-13-acetate-Induced inflammation and tumor promotion in mouse skin. Biol. Pharm. Bull. 1996, 19, 573–576.
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902.
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152.
- Zhang, D.H.; Lee, Y.K. Two-step process for ketocarotenoid production by a green alga, Chlorococcum sp. strain MA-1. Appl. Microbiol. Biotechnol. 2001, 55, 537–540.
- Zhang, D.H.; Lee, Y.K. Enhanced accumulation of secondary carotenoids in a mutant of the green alga, Chlorococcum sp. J. Appl. Phycol. 1997, 9, 459–463.
- Zhang, D.H.; Ng, M.L.; Phang, S.M. Composition and accumulation of secondary carotenoids in Chlorococcum sp. J. Appl. Phycol. 1997, 9, 147–155.
- Li, H.B.; Chen, F. Preparative Isolation and purification of astaxanthin from the green microalga Chlorococcum sp. by high-speed counter-current chromatography. In Algae and Their Biotechnological Potential; Chen, F., Jiang, Y., Eds.; Springer: Dordrecht, The Netherlands, 2001.
- Masojídek, J.; Torzillo, G.; Kopecký, J.; Koblížek, M.; Nidiaci, L.; Komenda, J.; Lukavská, A.; Sacchi, A. Changes in chlorophyll fluorescence quenching and pigment composition in the green alga Chlorococcum sp. grown under nitrogen deficiency and salinity stress. J. Appl. Phycol. 2000, 12, 417–426.
- Lang, I.; Hodač, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 124.
- Remias, D.; Wastian, H.; Lütz, C.; Leya, T. Insight into the biology and phylogeny of Chloromonas polyptera (Chlorophyta), an alga causing orange snow in Maritime Antarctica. Antarct. Sci. 2013, 25, 648–656.
- Hoham, R.; Remias, D. Snow and glacial algae: A review. J. Phycol. 2020, 56, 264–282.
- Koo, S.Y.; Cha, K.H.; Song, D.-G.; Chung, D.; Pan, C.-H. Optimization of pressurized liquid extraction of zeaxanthin from Chlorella ellipsoidea. J. Appl. Phycol. 2012, 24, 725–730.
- Lutz, S.; Anesio, A.M.; Raiswell, R.; Edwards, A.; Newton, R.J.; Gill, F.; Benning, L.G. The biogeography of red snow microbiomes and their role in melting arctic glaciers. Nat. Commun. 2016, 7, 11968.
- Procházková, L.; Remias, D.; Řezanka, T.; Nedbalová, L. Chloromonas nivalis subsp. tatrae, subsp. nov. (Chlamydomonadales, Chlorophyta): Re–examination of a snow alga from the High Tatra Mountains (Slovakia). Fottea 2018, 18, 1–18.
- Spijkerman, E.; Wacker, A.; Weithoff, G.; Leya, T. Elemental and fatty acid composition of snow algae in Arctic habitats. Front. Microbiol. 2012, 3, 380.
- Bar, E.; Rise, M.; Vishkautsan, M.; Arad, S. Pigment and structural changes in Chlorella zofingiensis upon light and nitrogen stress. J. Plant Physiol. 1995, 146, 527–534.
- Orosa, M.; Torres, E.; Fidalgo, P.; Abalde, J. Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 2000, 12, 553–556.
- Pelah, D.; Sintov, A.; Cohen, E. The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J. Microbiol. Biotechnol. 2004, 20, 483–486.
- Ip, P.F.; Chen, F. Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem. 2005, 40, 733–738.
- Ip, P.F.; Wong, K.-H.; Chen, F. Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process Biochem. 2004, 39, 1761–1766.
- Del Campo, J.A.; Rodriguez, H.; Moreno, J.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854.
- Ye, Y.; Huang, J.-C. Defining the biosynthesis of ketocarotenoids in Chromochloris zofingiensis. Plant Divers. 2020, 42, 61–66.
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515.
- Mulders, K.J.M.; Weesepoel, Y.; Bodenes, P.; Lamers, P.P.; Vincken, J.-P.; Martens, D.E.; Gruppen, H.; Wijffels, R.H. Nitrogen-depleted Chlorella zofingiensis produces astaxanthin, ketolutein and their fatty acid esters: A carotenoid metabolism study. J. Appl. Phycol. 2015, 27, 125–140.
- Orosa, M.; Valero, J.; Herrero, C. Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnol. Lett. 2001, 23, 1079–1085.
- Deenu, A.; Naruenartwongsakul, S.; Kim, S.M. Optimization and economic evaluation of ultrasound extraction of lutein from Chlorella vulgaris. Biotechnol. Bioprocess Eng. 2013, 18, 1151–1162.
- Osipova, S.; Dudareva, L.; Bondarenko, N.; Nasarova, A.; Sokolova, N.; Obolkina, L.; Glyzina, O.; Timoshkin, O. Temporal variation in fatty acid composition of Ulothrix zonata (Chlorophyta) from ice and benthic communities of Lake Baikal. Phycologia 2009, 48, 130–135.
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2007, 100, 656–661.
- Pollio, A.; Della Greca, M.; Monaco, P.; Pinto, G.; Previtera, L. Lipid composition of the acidophilic alga Dunaliella acidophila (Volvocales, Chlorophyta) I. Nonpolar lipids. Biochim. Biophys. Acta 1988, 963, 53–60.
- De los Reyes, C.; Ávila-Román, J.; Ortega, M.J.; de la Jara, A.; García-Mauriño, S.; Motilva, V.; Zubía, E. Oxylipins from the microalgae Chlamydomonas debaryana and Nannochloropsis gaditana and their activity as TNF-α inhibitors. Phytochemistry 2014, 102, 152–161.
- Fernández-Sevilla, J.M.; Acién-Fernández, F.; Molina-Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40.
- Bux, F. (Ed.) Biotechnological Applications of Microalgae Biodiesel and Value-Added Products; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2013.
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Varga, M.Á.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59.
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Varga, M.Á.; Rivas, J.; Guerrero, M.G. Lutein production by Muriellopsis sp. in an outdoor tubular photobiorector. J. Biotechnol. 2001, 81, 289–295.
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174.
- Bigogno, C.; Khozin-Goldberg, I.; Boussiba, S.; Vonshak, A.; Cohen, Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 2002, 60, 497–503.
- Abu-Ghosh, S.; Pal-Nath, D.; Markovitch, D.; Solovchenko, A.; Didi-Cohen, S.; Portugal, I.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. A novel source of dihomo-γ-linolenic acid: Possibilities and limitations of DGLA production in the high-density cultures of the Δ5 desaturase-mutant microalga Lobosphaera incisa. Eur. J. Lipid Sci. Technol. 2015, 117, 760–766.
- Lee, S.; Lim, S.R.; Jeong, D.G.; Kim, J.H. Characterization of an oleaginous unicellular green microalga, Lobosphaera incisa (Reisigl, 1964) Strain K-1, isolated from a tidal flat in the Yellow Sea, Republic of Korea. Front. Microbiol. 2018, 9, 2159.
- Bigogno, C.; Khozin-Goldberg, I.; Cohen, Z. Accumulation of arachidonic acid-rich triacylglycerols in the microalga Parietochloris incisa (Trebuxiophyceae, Chlorophyta). Phytochemistry 2002, 60, 135–143.
- Satoh, A.; Kato, M.; Yamato, K.; Ishibashi, M.; Sekiguchi, H.; Kurano, N.; Miyachi, S. Characterization of the lipid accumulation in a new microalgal species, Pseudochoricystis ellipsoidea (Trebouxiophyceae). J. Jpn. Inst. Energy 2010, 89, 909–913.
- Procházková, L.; Leya, T.; Křížková, H.; Nedbalová, L. Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): The taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow. FEMS Microbiol. Ecol. 2019, 95, fiz064.
- Chan, M.-C.; Ho, S.-H.; Lee, D.-J.; Chen, C.-Y.; Huang, C.-C.; Chang, J.-S. Characterization, extraction and purification of lutein produced by an indigenous microalga Scenedesmus obliquus CNW-N. Biochem. Eng. J. 2013, 78, 24–31.
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196.
- Remias, D.; Albert, A.; Lütz, C. Effects of realistically simulated, elevated UV irradiation on photosynthesis and pigment composition of the alpine snow alga Chlamydomonas nivalis and the arctic soil alga Tetracystis sp. (Chlorophyceae). Photosynthetica 2010, 48, 269–277.
- Da Silva, M.E.T.; Martins, M.A.; de Oliveira Leite, M.; Milião, G.L.; dos Reis Coimbra, J.S. Microalga Scenedesmus obliquus: Extraction of bioactive compounds and antioxidant activity. Rev. Cienc. Agron. 2021, 52, e20196848.
- Da Silva, M.E.T.; de Paula Correa, K.; Martins, M.A.; da Matta, S.L.P.; Martino, H.S.D.; dos Reis Coimbra, J.S. Food safety, hypolipidemic and hypoglycemic activities, and in vivo protein quality of microalga Scenedesmus obliquus in Wistar rats. J. Funct. Foods 2020, 65, 103711.
- Rocha, D.N.; Martins, M.A.; Soares, J.; Vaz, M.G.M.V.; de Oliveira Leite, M.; Covell, L.; Mendes, L.B.B. Combination of trace elements and salt stress in different cultivation modes improves the lipid productivity of Scenedesmus spp. Bioresour. Technol. 2019, 289, 121644.
- Wiltshire, K.H.; Boersma, M.; Möller, A.; Buhtz, H. Extraction of pigments and fatty acids from the green alga Scenedesmus obliquus (Chlorophyceae). Aquatic Ecol. 2000, 34, 119–126.
- Qin, S.; Liu, G.-X.; Hu, Z.-Y. The accumulation and metabolism of astaxanthin in Scenedesmus obliquus (Chlorophyceae). Process Biochem. 2008, 43, 795–802.
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282.
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar Drugs 2020, 18, 323.
- Hofbauer, W.K. Toxic or otherwise harmful algae and the built environment. Toxins 2021, 13, 465.
