Corrosion is generally viewed as the destructive consequence of chemical reaction between a metal or metal alloy and its environment that contains corrosive agents. Compared to inorganic corrosion inhibitors, the organic ones are less toxic, can be also used at low concentrations, and have a better film-forming ability. The adsorption properties of organic corrosion inhibitors are primarily linked to the presence of both π-electrons from aromatic rings and heteroatoms in the molecular structures. Indeed, organic compounds containing nitrogen, phosphorus, sulfur, and oxygen atoms have been extensively investigated as corrosion inhibitors of metals and their alloys in acidic environments. In this respect, triazole derivatives are the most used nitrogen-containing organic inhibitors, in particular the family of 1,2,3-triazoles prepared under click chemistry regime by the prolific copper-catalyzed azide cycloaddition reactions.
- triazoles
- click chemistry
- corrosion inhibitor
- metal
- metal alloys
- mechanism of inhibition.
1. Synthesis of 1,2,3-triazoles
The [3+2] cycloaddition reaction between organic azides and alkynes catalyzed by copper(I) (CuAAC) is the most applicable and best known synthetic method for the selective preparation of 1,4-disubstituted-1,2,3-triazole derivatives. The first described preparative route to obtain triazole compounds was reported in the 1960s by Huisgen et al. [1]. This process requires high temperatures, typically solvent (toluene or carbon tetrachloride) reflux conditions, and prolonged reaction periods generally between 12 and 60 h. Under these thermal conditions, the two possible regioisomers (1,4- and 1,5-disubstituted-1,2,3-triazole derivatives) are formed in an equimolar proportion (top route in Figure 1). All this changed in 2002 when Sharpless, Fokin, and their colleagues at the Scripps Research Institute (at La Jolla) and Meldal’s group at the Carlsberg Laboratory (at Copenhagen) [2][3][4] worked in parallel on this reaction and independently discovered that copper(I) catalyzed the azide-alkyne cycloaddition reaction (CuAAC), providing a regioselectivity synthesis of only 1,4-disubstituted-1,2,3-triazoles in very high yields (bottom route in Figure 1).
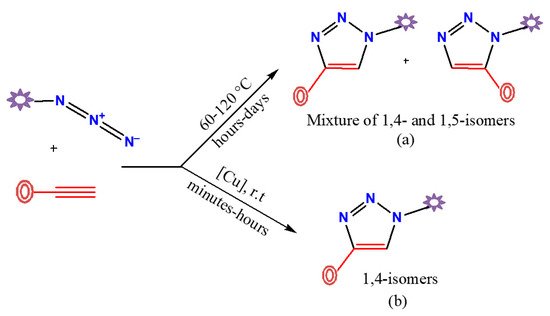
Figure 1. Non-regioselective (a) and regioselective (b) preparative routes of disubstituted-1,2,3-triazole derivatives by azide-alkyne cycloaddition reaction.
The continued research and development of new corrosion inhibitors is a matter of great importance and interest, both commercially and scientifically. Within this frame, several types of inhibitors have been developed and used to effectively inhibit the corrosion of metals; they can be classified as either inorganic or organic compounds. Typically, inorganic corrosion inhibitors have either anodic or cathodic action [5], while organic corrosion inhibitors have both anodic and cathodic roles (mixed-type), as well as a protective action by film adsorption (Figure 2). It is well established that the organic corrosion inhibitors are more effective and less expensive than the inorganic ones [6]. In this context, 1,2,3-triazole derivatives, which were initially studied as biologically active compounds and relevant medicinal compounds [7][8][9][10][11][12], have been reported as corrosion inhibitors for steels [13], copper, aluminum, and their alloys in corrosive media [14][15].
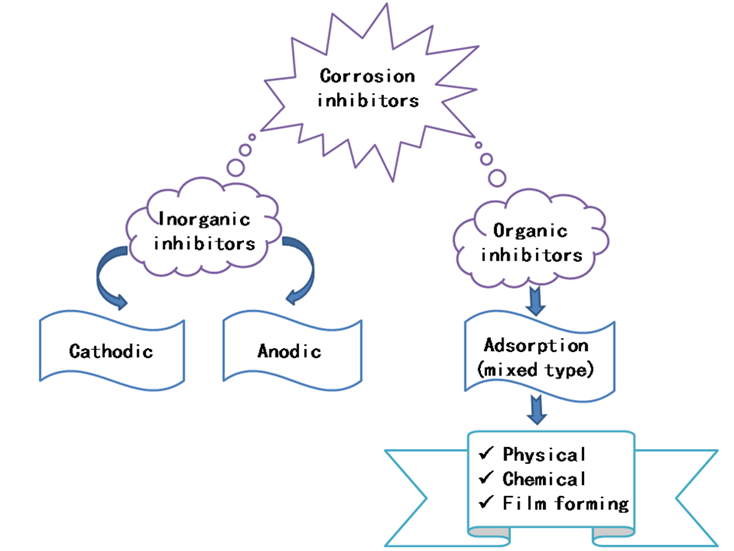
Figure 2. Classification of organic and inorganic corrosion inhibitors.
2. Mechanism of Corrosion Inhibition
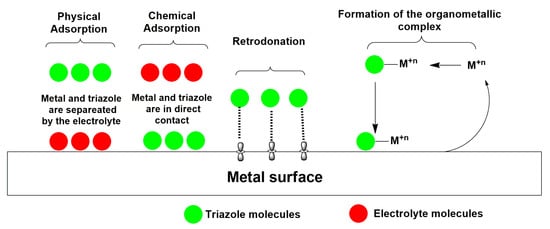
Figure 3. Adsorption behavior of the triazole inhibitors on the metal surface.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23010016
References
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. 1963, 2(10), 565–598.
- Kolb, H.C.; Finn, M. G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2012.
- Rostovtsev, V.V.; Green, L.G.; Fokin, V. V.; Sharpless, K.B. A. Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596-2599.
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regioselective Copper(I)-Catalyzed 1,3, Dipolar Cycloaddition of Terminal Alkynes to Azides. J. Org. Chem. Chem. 2002, 67, 3057-3062.
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2002, 108, 2952-3015.
- Vishnudevan, M.; Thangavel, K. A comparative study of inorganic versus organic corrosion inhibitors for mitigation of steel in chloride contaminated alkaline solution. Indian. J. Chem. Technol. 2007, 14, 22–28.
- Asif, M. Antivral and antiparasitic activities of various substituted triazole derivatives: A mini review. Chem. Int. 2015,1(2) 71-80.
- Iwasaki, M.; Yorimitsu, H.; Oshima, K. Microwave-Assisted Palladium-Catalyzed Direct Arylation of 1,4-Disubstituted 1,2,3-Triazoles with Aryl Chlorides. Chem. Asian. J. 2007, 2(11), 1430–1435.
- Deswal, S.; Naveen, Tittal, R.K.; Ghule Vikas, D.; Lal, K.; Kumar, A. 5-Fluoro-1H-indole-2,3-dione-triazoles- synthesis, biological activity, molecular docking, and DFT study. J. Mol. Struct. 2020, 127982.
- Aziz Ali, A.; Gogoi, D.; Chaliha, A.K.; Buragohain, A.K.; Trivedi, P.; Saikia, P.J.; Sarma, D. Synthesis and biological evaluation of novel 1,2,3-triazole derivatives as anti-tubercular agents. Bio. Med. Chem. Lett. 2017, 27(16), 3698–3703.
- Reddyrajula, R.; Dalimba, U. The bioisosteric modification of pyrazinamide derivatives led to potent antitubercular agents: Synthesis via click approach and molecular docking of pyrazine-1,2,3-triazoles. Bioorg. Med. Chem. Lett. 2019, 126846.
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: A Promising Antitubercular Agent. Chem. Bio. Drug. Des. 2015, 86(4), 410–423.
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41.
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2017, 13(1), 740-771.
- Ofoegbu, S.U.; Galvão, T.L.P.; Gomes, J.R.B.; Tedim, J.; Nogueira, H.I.S.; Ferreira, M. G.S.; Zheludkevich, M.L. Corrosion inhibition of copper in aqueous chloride solution by 1H-1,2,3-triazole and 1,2,4-triazole and their combinations: electrochemical, Raman and theoretical studies. Phys. Chem. Chem. Phys. 2017,19(8), 6113–6129.
- Esimone, M.P.; Grundmeier, G.; Gordillo, G.; Simison, S.N. Amphiphilic amido-amine as an effective corrosion inhibitor for mild steel exposed to CO2 saturated solution: Polarization, EIS and PM-IRRAS studies. Electrochim. Acta. 2011, 56(8), 2990–2998.
- Li, X.; Deng, S.; Fu, H.; Li, T. Adsorption and inhibition effect of 6-benzyl aminopurine on cold rolled steel in 1.0 M HCl. Electrochim. Acta. 2009, 54(16), 4089–4098.
- Şahin, M.; Bilgiç, S.; Yılmaz, H. The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl. Surf. Sci. 2002, 195(1-4), 1–7.
- Verma, C.B.; Quraishi, M.A.; Singh, A. 2-Aminobenzene-1,3-dicarbonitriles as green corrosion inhibitor for mild steel in 1 M HCl: Electrochemical, thermodynamic, surface and quantum chemical investigation. J. Taiwan. Inst. Chem. Eng. 2015, 49, 229–239.
- Keleş, H.; Keleş, M.; Dehri, İ.; Serindağ, O. The inhibitive effect of 6-amino-m-cresol and its Schiff base on the corrosion of mild steel in 0.5 M HCI medium. Mater. Chem. Phys. 2008, 112(1), 173–179.
- Deng, S.; Li, X.; Xie, X. Hydroxymethyl urea and 1,3-bis(hydroxymethyl) urea as corrosion inhibitors for steel in HCl solution. Corros. Sci. 2014, 80, 276–289.
- Hrimla, M.; Bahsis, L.; Boutouil, A.; Laamari, M. R.; Julve, M.; Stiriba, S.-E. Corrosion inhibition performance of a structurally well-defined 1, 2, 3-triazole derivative on mild steel-hydrochloric acid interface. J. Mol. Struct. 2021, 1231, 129895.
- Hrimla, M.; Achour, Y.; Bahsis, L.; Laamari, M. R.; Anane, H.; Julve, M.; Stiriba, S.-E. Systematic investigation of the adsorption and inhibition properties of a new clickable 1,2,3-triazole compound for mild steel in 1 M HCl medium. ChemistrySelect 2021, 6, 12895-12913.
