Calcium signaling plays important roles in physiological and pathological conditions, including cutaneous melanoma, the most lethal type of skin cancer. Intracellular calcium concentration ([Ca2+]i), cell membrane calcium channels, calcium related proteins (S100 family, E-cadherin, and calpain), and Wnt/Ca2+ pathways are related to melanogenesis and melanoma tumorigenesis and progression. Calcium signaling influences the melanoma microenvironment, including immune cells, extracellular matrix (ECM), the vascular network, and chemical and physical surroundings. Other ionic channels, such as sodium and potassium channels, are engaged in calcium-mediated pathways in melanoma. Calcium signaling serves as a promising pharmacological target in melanoma treatment, and its dysregulation might serve as a marker for melanoma prediction.
- calcium
- melanoma
- progression
- melanoma microenvironment
- mitochondria
1. Calcium Signaling in Melanoma Progression
1.1. [Ca2+]i Oscillation Influences Melanoma Progression
1.2. Calcium Channels Are Involved in Melanoma Progression
1.3. Ca2+ Signaling Influences Melanoma Progression through the Change of Morphological and Phenotypical Changes
1.4. Calcium-Related Pathways Participate in Melanoma Progression
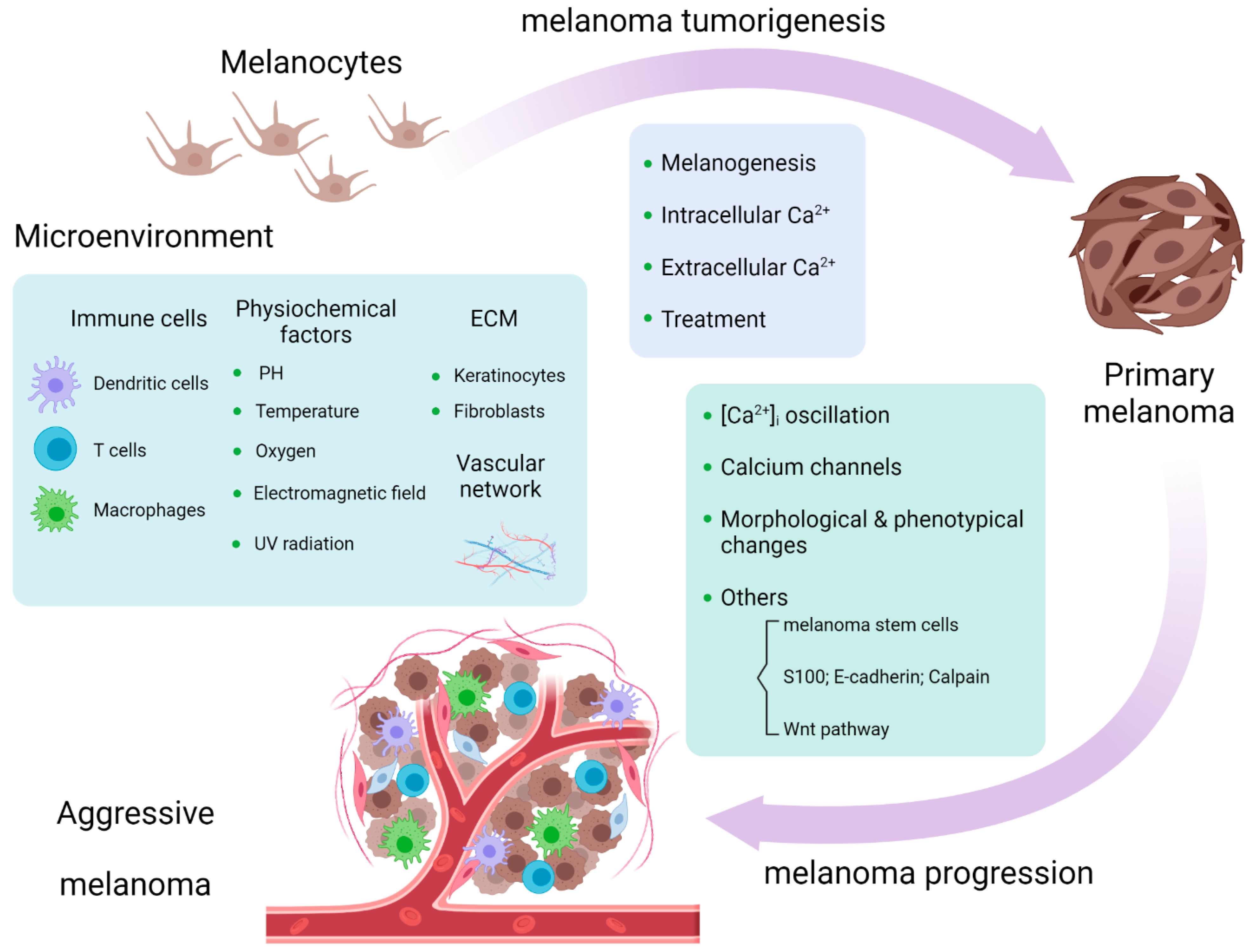 Figure 1. Calcium signaling is involved in melanoma tumorigenesis and progression and melanoma microenvironment [33].
Figure 1. Calcium signaling is involved in melanoma tumorigenesis and progression and melanoma microenvironment [33].2. Calcium Signaling in Melanoma Microenvironment
2.1. Immune Cells
2.2. ECM and Vascular Network
2.3. Physical and Chemical Surroundings
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031010
References
- Martinez-Zaguilan, R.; Martinez, G.M.; Gomez, A.; Hendrix, M.J.C.; Gillies, R.J. Distinct regulation of pH(in) and (in) in human melanoma cells with different metastatic potential. J. Cell Physiol. 1998, 176, 196–205.
- Baljinnyam, E.; Umemura, M.; De Lorenzo, M.S.; Xie, L.H.; Nowycky, M.; Iwatsubo, M.; Chen, S.; Goydos, J.S.; Iwatsubo, K. Gbetagamma subunits inhibit Epac-induced melanoma cell migration. BMC Cancer 2011, 11, 256.
- Baljinnyam, E.; De Lorenzo, M.S.; Xie, L.H.; Iwatsubo, M.; Chen, S.; Goydos, J.S.; Nowycky, M.C.; Iwatsubo, K. Exchange protein directly activated by cyclic AMP increases melanoma cell migration by a Ca2+-dependent mechanism. Cancer Res. 2010, 70, 5607–5617.
- Arozarena, I.; Sanchez-Laorden, B.; Packer, L.; Hidalgo-Carcedo, C.; Hayward, R.; Viros, A.; Sahai, E.; Marais, R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell 2011, 19, 45–57.
- Kosnopfel, C.; Sinnberg, T.; Sauer, B.; Niessner, H.; Muenchow, A.; Fehrenbacher, B.; Schaller, M.; Mertens, P.R.; Garbe, C.; Thakur, B.K.; et al. Tumour Progression Stage-Dependent Secretion of YB-1 Stimulates Melanoma Cell Migration and Invasion. Cancers 2020, 12, 2328.
- Gelis, L.; Jovancevic, N.; Bechara, F.G.; Neuhaus, E.M.; Hatt, H. Functional expression of olfactory receptors in human primary melanoma and melanoma metastasis. Exp. Dermatol. 2017, 26, 569–576.
- D’Mello, S.A.; Joseph, W.R.; Green, T.N.; Leung, E.Y.; During, M.J.; Finlay, G.J.; Baguley, B.C.; Kalev-Zylinska, M.L. Selected GRIN2A mutations in melanoma cause oncogenic effects that can be modulated by extracellular glutamate. Cell Calcium 2016, 60, 384–395.
- Choi, K.Y.; Chang, K.; Pickel, J.M.; Badger, J.D., 2nd; Roche, K.W. Expression of the metabotropic glutamate receptor 5 (mGluR5) induces melanoma in transgenic mice. Proc. Natl. Acad. Sci. USA 2011, 108, 15219–15224.
- Umemura, M.; Baljinnyam, E.; Feske, S.; De Lorenzo, M.S.; Xie, L.H.; Feng, X.; Oda, K.; Makino, A.; Fujita, T.; Yokoyama, U.; et al. Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS ONE 2014, 9, e89292.
- D’Amore, A.; Hanbashi, A.A.; Di Agostino, S.; Palombi, F.; Sacconi, A.; Voruganti, A.; Taggi, M.; Canipari, R.; Blandino, G.; Parrington, J.; et al. Loss of Two-Pore Channel 2 (TPC2) Expression Increases the Metastatic Traits of Melanoma Cells by a Mechanism Involving the Hippo Signalling Pathway and Store-Operated Calcium Entry. Cancers 2020, 12, 2391.
- Hegedus, L.; Garay, T.; Molnar, E.; Varga, K.; Bilecz, A.; Torok, S.; Padanyi, R.; Paszty, K.; Wolf, M.; Grusch, M.; et al. The plasma membrane Ca(2+) pump PMCA4b inhibits the migratory and metastatic activity of BRAF mutant melanoma cells. Int. J. Cancer 2017, 140, 2758–2770.
- Naffa, R.; Vogel, L.; Hegedus, L.; Paszty, K.; Toth, S.; Kelemen, K.; Singh, N.; Remenyi, A.; Kallay, E.; Cserepes, M.; et al. P38 MAPK Promotes Migration and Metastatic Activity of BRAF Mutant Melanoma Cells by Inducing Degradation of PMCA4b. Cells 2020, 9, 1209.
- Long, T.; Su, J.; Tang, W.; Luo, Z.; Liu, S.; Liu, Z.; Zhou, H.; Qi, M.; Zeng, W.; Zhang, J.; et al. A novel interaction between calcium-modulating cyclophilin ligand and Basigin regulates calcium signaling and matrix metalloproteinase activities in human melanoma cells. Cancer Lett. 2013, 339, 93–101.
- Maiques, O.; Barcelo, C.; Panosa, A.; Pijuan, J.; Orgaz, J.L.; Rodriguez-Hernandez, I.; Matas-Nadal, C.; Tell, G.; Vilella, R.; Fabra, A.; et al. T-type calcium channels drive migration/invasion in BRAFV600E melanoma cells through Snail1. Pigment Cell Melanoma Res. 2018, 31, 484–495.
- Yang, Y.; Luo, Z.; Hao, Y.; Ba, W.; Wang, R.; Wang, W.; Ding, X.; Li, C. mTOR-mediated Na(+)/Ca(2+) exchange affects cell proliferation and metastasis of melanoma cells. Biomed. Pharmacother. 2017, 92, 744–749.
- Schmidt, J.; Friebel, K.; Schonherr, R.; Coppolino, M.G.; Bosserhoff, A.K. Migration-associated secretion of melanoma inhibitory activity at the cell rear is supported by KCa3.1 potassium channels. Cell Res. 2010, 20, 1224–1238.
- Jia, Q.; Hu, S.; Jiao, D.; Li, X.; Qi, S.; Fan, R. Synaptotagmin-4 promotes dendrite extension and melanogenesis in alpaca melanocytes by regulating Ca(2+) influx via TRPM1 channels. Cell Biochem. Funct. 2020, 38, 275–282.
- Sun, J.; Lu, F.; He, H.; Shen, J.; Messina, J.; Mathew, R.; Wang, D.; Sarnaik, A.A.; Chang, W.C.; Kim, M.; et al. STIM1- and Orai1-mediated Ca(2+) oscillation orchestrates invadopodium formation and melanoma invasion. J. Cell. Biol. 2014, 207, 535–548.
- Sun, J.; Lin, S.; Keeley, T.; Yang, S. Disseminating Melanoma Cells Surf on Calcium Waves. Mol. Cell. Oncol. 2015, 2, e1002714.
- Kim, T.H.; Gill, N.K.; Nyberg, K.D.; Nguyen, A.V.; Hohlbauch, S.V.; Geisse, N.A.; Nowell, C.J.; Sloan, E.K.; Rowat, A.C. Cancer cells become less deformable and more invasive with activation of beta-adrenergic signaling. J. Cell Sci. 2016, 129, 4563–4575.
- Meghnani, V.; Vetter, S.W.; Leclerc, E. RAGE overexpression confers a metastatic phenotype to the WM115 human primary melanoma cell line. Biochim. Biophys. Acta 2014, 1842, 1017–1027.
- Terrie, E.; Coronas, V.; Constantin, B. Role of the calcium toolkit in cancer stem cells. Cell Calcium 2019, 80, 141–151.
- Neves de Oliveira, B.H.; Dalmaz, C.; Zeidan-Chulia, F. Network-Based Identification of Altered Stem Cell Pluripotency and Calcium Signaling Pathways in Metastatic Melanoma. Med. Sci. 2018, 6, 23.
- Bettum, I.J.; Gorad, S.S.; Barkovskaya, A.; Pettersen, S.; Moestue, S.A.; Vasiliauskaite, K.; Tenstad, E.; Oyjord, T.; Risa, O.; Nygaard, V.; et al. Metabolic reprogramming supports the invasive phenotype in malignant melanoma. Cancer Lett. 2015, 366, 71–83.
- Zhu, L.; Ito, T.; Nakahara, T.; Nagae, K.; Fuyuno, Y.; Nakao, M.; Akahoshi, M.; Nakagawa, R.; Tu, Y.; Uchi, H.; et al. Upregulation of S100P, receptor for advanced glycation end products and ezrin in malignant melanoma. J. Dermatol. 2013, 40, 973–979.
- Venza, M.; Visalli, M.; Catalano, T.; Biondo, C.; Beninati, C.; Teti, D.; Venza, I. DNA methylation-induced E-cadherin silencing is correlated with the clinicopathological features of melanoma. Oncol. Rep. 2016, 35, 2451–2460.
- Wu, L.; Zhu, L.; Li, Y.; Zheng, Z.; Lin, X.; Yang, C. LncRNA MEG3 promotes melanoma growth, metastasis and formation through modulating miR-21/E-cadherin axis. Cancer Cell Int. 2020, 20, 12.
- Rodriguez, M.; Aladowicz, E.; Lanfrancone, L.; Goding, C.R. Tbx3 represses E-cadherin expression and enhances melanoma invasiveness. Cancer Res. 2008, 68, 7872–7881.
- Raimbourg, Q.; Perez, J.; Vandermeersch, S.; Prignon, A.; Hanouna, G.; Haymann, J.P.; Baud, L.; Letavernier, E. The calpain/calpastatin system has opposing roles in growth and metastatic dissemination of melanoma. PLoS ONE 2013, 8, e60469.
- Weeraratna, A.T. A wnt-er wonderland—The complexity of wnt signaling in melanoma. Cancer Metast. Rev. 2005, 24, 237–250.
- O’Connell, M.P.; Fiori, J.L.; Baugher, K.M.; Indig, F.E.; French, A.D.; Camilli, T.C.; Frank, B.P.; Earley, R.; Hoek, K.S.; Hasskamp, J.H.; et al. Wnt5A activates the calpain-mediated cleavage of filamin A. J. Investig. Dermatol. 2009, 129, 1782–1789.
- Witze, E.S.; Connacher, M.K.; Houel, S.; Schwartz, M.P.; Morphew, M.K.; Reid, L.; Sacks, D.B.; Anseth, K.S.; Ahn, N.G. Wnt5a directs polarized calcium gradients by recruiting cortical endoplasmic reticulum to the cell trailing edge. Dev. Cell 2013, 26, 645–657.
- Adapted from “Tumor Microenvironment”, by BioRender.com. Available online: https://app.biorender.com/biorender-templates (accessed on 19 December 2021).
- Singh, K.; Rosenberg, P. Anti-tumour activity and store operated calcium entry: New roles in immunology. EMBO Mol. Med. 2013, 5, 1297–1299.
- Kim, K.D.; Bae, S.; Capece, T.; Nedelkovska, H.; de Rubio, R.G.; Smrcka, A.V.; Jun, C.D.; Jung, W.; Park, B.; Kim, T.I.; et al. Targeted calcium influx boosts cytotoxic T lymphocyte function in the tumour microenvironment. Nat. Commun. 2017, 8, 15365.
- Mookerjee-Basu, J.; Hooper, R.; Gross, S.; Schultz, B.; Go, C.K.; Samakai, E.; Ladner, J.; Nicolas, E.; Tian, Y.; Zhou, B.; et al. Suppression of Ca(2+) signals by EGR4 controls Th1 differentiation and anti-cancer immunity in vivo. EMBO Rep. 2020, 21, e48904.
- Truta-Feles, K.; Lagadari, M.; Lehmann, K.; Berod, L.; Cubillos, S.; Piehler, S.; Herouy, Y.; Barz, D.; Kamradt, T.; Maghazachi, A.; et al. Histamine modulates gammadelta-T lymphocyte migration and cytotoxicity, via Gi and Gs protein-coupled signalling pathways. Br. J. Pharmacol. 2010, 161, 1291–1300.
- Key, P.N.; Germino, J.; Yang, L.; Piersma, S.J.; Tripathy, S.K. Chronic Ly49H Receptor Engagement in vivo Decreases NK Cell Response to Stimulation Through ITAM-Dependent and Independent Pathways Both in vitro and in vivo. Front. Immunol. 2019, 10, 1692.
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896.
- Hollander, L.; Guo, X.; Velazquez, H.; Chang, J.; Safirstein, R.; Kluger, H.; Cha, C.; Desir, G.V. Renalase Expression by Melanoma and Tumor-Associated Macrophages Promotes Tumor Growth through a STAT3-Mediated Mechanism. Cancer Res. 2016, 76, 3884–3894.
- Hakonen, E.; Chandra, V.; Fogarty, C.L.; Yu, N.Y.; Ustinov, J.; Katayama, S.; Galli, E.; Danilova, T.; Lindholm, P.; Vartiainen, A.; et al. MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia 2018, 61, 2202–2214.
- Peled, M.; Bar-Lev, T.H.; Talalai, E.; Aspitz, H.Z.; Daniel-Meshulam, I.; Bar, J.; Kamer, I.; Ofek, E.; Mor, A.; Onn, A. Mesencephalic astrocyte-derived neurotrophic factor is secreted from interferon-gamma-activated tumor cells through ER calcium depletion. PLoS ONE 2021, 16, e0250178.
- Tremble, L.F.; Heffron, C.; Forde, P.F. The effect of calcium electroporation on viability, phenotype and function of melanoma conditioned macrophages. Sci. Rep. 2020, 10, 20645.
- Falk, H.; Matthiessen, L.W.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018, 57, 311–319.
- He, L.; Zhang, Y.; Ma, G.; Tan, P.; Li, Z.; Zang, S.; Wu, X.; Jing, J.; Fang, S.; Zhou, L.; et al. Near-infrared photoactivatable control of Ca(2+) signaling and optogenetic immunomodulation. Elife 2015, 4, e10024.
- Ramirez-Moreno, I.G.; Ibarra-Sanchez, A.; Castillo-Arellano, J.I.; Blank, U.; Gonzalez-Espinosa, C. Mast Cells Localize in Hypoxic Zones of Tumors and Secrete CCL-2 under Hypoxia through Activation of L-Type Calcium Channels. J. Immunol. 2020, 204, 1056–1068.
- Shahan, T.A.; Fawzi, A.; Bellon, G.; Monboisse, J.C.; Kefalides, N.A. Regulation of tumor cell chemotaxis by type IV collagen is mediated by a Ca(2+)-dependent mechanism requiring CD47 and the integrin alpha(V)beta(3). J. Biol. Chem. 2000, 275, 4796–4802.
- Hodgson, L.; Dong, C. (i) as a potential downregulator of alpha(2)beta(1)-integrin-mediated A2058 tumor cell migration to type IV collagen. Am. J. Physiol.-Cell Physiol. 2001, 281, C106–C113.
- Huang, H.C.; Shi, G.Y.; Jiang, S.J.; Shi, C.S.; Wu, C.M.; Yang, H.Y.; Wu, H.L. Thrombomodulin-mediated cell adhesion: Involvement of its lectin-like domain. J. Biol. Chem. 2003, 278, 46750–46759.
- Krenzer, S.; Peterziel, H.; Mauch, C.; Blaber, S.I.; Blaber, M.; Angel, P.; Hess, J. Expression and function of the kallikrein-related peptidase 6 in the human melanoma microenvironment. J. Investig. Dermatol. 2011, 131, 2281–2288.
- Chung, H.; Jung, H.; Jho, E.H.; Multhaupt, H.A.B.; Couchman, J.R.; Oh, E.S. Keratinocytes negatively regulate the N-cadherin levels of melanoma cells via contact-mediated calcium regulation. Biochem. Biophys. Res. Commun. 2018, 503, 615–620.
- Slater, M.; Scolyer, R.A.; Gidley-Baird, A.; Thompson, J.F.; Barden, J.A. Increased expression of apoptotic markers in melanoma. Melanoma. Res. 2003, 13, 137–145.
- Li, G.; Satyamoorthy, K.; Meier, F.; Berking, C.; Bogenrieder, T.; Herlyn, M. Function and regulation of melanoma-stromal fibroblast interactions: When seeds meet soil. Oncogene 2003, 22, 3162–3171.
- Ekstrom, E.J.; Bergenfelz, C.; von Bulow, V.; Serifler, F.; Carlemalm, E.; Jonsson, G.; Andersson, T.; Leandersson, K. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer 2014, 13, 88.
- Favia, A.; Pafumi, I.; Desideri, M.; Padula, F.; Montesano, C.; Passeri, D.; Nicoletti, C.; Orlandi, A.; Del Bufalo, D.; Sergi, M.; et al. NAADP-Dependent Ca(2+) Signaling Controls Melanoma Progression, Metastatic Dissemination and Neoangiogenesis. Sci. Rep. 2016, 6, 18925.
- Zhang, W.; Zhou, P.; Meng, A.; Zhang, R.; Zhou, Y. Down-regulating Myoferlin inhibits the vasculogenic mimicry of melanoma via decreasing MMP-2 and inducing mesenchymal-to-epithelial transition. J. Cell. Mol. Med. 2018, 22, 1743–1754.
- Vartanian, A.; Stepanova, E.; Grigorieva, I.; Solomko, E.; Belkin, V.; Baryshnikov, A.; Lichinitser, M. Melanoma vasculogenic mimicry capillary-like structure formation depends on integrin and calcium signaling. Microcirculation 2011, 18, 390–399.
- Oliver, V.K.; Patton, A.M.; Desai, S.; Lorang, D.; Libutti, S.K.; Kohn, E.C. Regulation of the pro-angiogenic microenvironment by carboxyamido-triazole. J. Cell. Physiol. 2003, 197, 139–148.
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Tramm, T.; Eriksen, J.; Gehl, J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012, 72, 1336–1341.
- Staresinic, B.; Jesenko, T.; Kamensek, U.; Krog Frandsen, S.; Sersa, G.; Gehl, J.; Cemazar, M. Effect of calcium electroporation on tumour vasculature. Sci. Rep. 2018, 8, 9412.
- Boda-Heggemann, J.; Regnier-Vigouroux, A.; Franke, W.W. Beyond vessels: Occurrence and regional clustering of vascular endothelial (VE-)cadherin-containing junctions in non-endothelial cells. Cell Tissue Res. 2009, 335, 49–65.
- Peng, H.H.; Hodgson, L.; Henderson, A.J.; Dong, C. Involvement of phospholipase C signaling in melanoma cell-induced endothelial junction disassembly. Front Biosci. 2005, 10, 1597–1606.
- Peng, H.H.; Dong, C. Systemic Analysis of Tumor Cell-Induced Endothelial Calcium Signaling and Junction Disassembly. Cell. Mol. Bioeng. 2009, 2, 375–385.
- Kato, Y.; Ozawa, S.; Tsukuda, M.; Kubota, E.; Miyazaki, K.; St-Pierre, Y.; Hata, R. Acidic extracellular pH increases calcium influx-triggered phospholipase D activity along with acidic sphingomyelinase activation to induce matrix metalloproteinase-9 expression in mouse metastatic melanoma. FEBS J. 2007, 274, 3171–3183.
- Noguchi, F.; Inui, S.; Fedele, C.; Shackleton, M.; Itami, S. Calcium-Dependent Enhancement by Extracellular Acidity of the Cytotoxicity of Mitochondrial Inhibitors against Melanoma. Mol. Cancer Ther. 2017, 16, 936–947.
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.K. Acidification is an Essential Process of Cold Atmospheric Plasma and Promotes the Anti-Cancer Effect on Malignant Melanoma Cells. Cancers 2019, 11, 671.
- Hung, W.C.; Yang, J.R.; Yankaskas, C.L.; Wong, B.S.; Wu, P.H.; Pardo-Pastor, C.; Serra, S.A.; Chiang, M.J.; Gu, Z.; Wirtz, D.; et al. Confinement Sensing and Signal Optimization via Piezo1/PKA and Myosin II Pathways. Cell Rep. 2016, 15, 1430–1441.
- Buckner, C.A.; Buckner, A.L.; Koren, S.A.; Persinger, M.A.; Lafrenie, R.M. Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS ONE 2015, 10, e0124136.
- Yu, S.; Li, C.; Ding, Y.; Huang, S.; Wang, W.; Wu, Y.; Wang, F.; Wang, A.; Han, Y.; Sun, Z.; et al. Exploring the ‘cold/hot’ properties of traditional Chinese medicine by cell temperature measurement. Pharm. Biol. 2020, 58, 208–218.
- Nam, J.H.; Lee, D.U. Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J. Dermatol. Sci. 2016, 84, 305–313.
- Slominski, A.T.; Brozyna, A.A.; Zmijewski, M.A.; Jozwicki, W.; Jetten, A.M.; Mason, R.S.; Tuckey, R.C.; Elmets, C.A. Vitamin D signaling and melanoma: Role of vitamin D and its receptors in melanoma progression and management. Lab. Investig. 2017, 97, 706–724.
- Kleszczynski, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Bohm, M.; Slominski, A.T. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 3786.
