Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
|
Others
Alzheimer’s disease (AD) incidence is increasing worldwide at an alarming rate. Considering this increase, prevention efforts, stemming from scientific research, health education, and public policies, are critical. Clinical studies evidenced that healthy lifestyles along with natural multitarget and disease-modifying agents have a preventative impact on AD or mitigate symptoms in diagnosed patients.
- AD prevention
- interventional approaches toward Alzheimer´s treatment
- nutraceutical compounds
- mechanisms of action in AD therapy
1. Introduction
Alzheimer’s Disease (AD) is a progressive neurodegenerative disease characterized by cognitive deterioration, mood alterations, and neuropsychiatric disorders. There are more than 52 million people worldwide affected by AD (Alzheimer Report WHO), while most of pharmacological agents have only some palliative actions [1]. Besides the deteriorating effects of AD on human health and the quality of life of elderly persons, there is also a tremendous economic impact associated with the disease. Economical cost of AD in the world reaches one billion dollars a year [2]. AD is not only a medical issue and a puzzle for society but also linked with public policies in the search for quality of life of patients and protection of caregivers for AD patients. In this context, we are promoting integrative action from basic and translational research to development of innovative technologies and actions in favor of caregivers.
Neuroinflammation is one of the major causes of Alzheimer’s disease. The mechanisms on how the inflammatory process occurs in the human brain starts with the so named “damage signals”, which interfere with the cross-talks neuron-glia. Consequently, activated microglia produce NFkB, leading the synthesis of proinflammatory mediators that finally signal on neuronal receptors, with reactivation of proteins kinases responsible for tau hyperphosphorylation. In a search of nutraceutical bioactive principles, we can find compounds with tau antiaggregant activity, as well as compounds with antioxidative and anti-inflammatory activities [3,4,5].
In this context, and considering the explosive increase in AD incidence, the path to AD prevention appears as a most promising avenue to control the spread of this disease [6]. Healthy lifestyles, along with several nonpharmacological actions, were demonstrated through clinical studies to prevent manifestations of the disease and even mitigate the symptoms of diagnosed AD patients [7]. These actions include cognitive and sensory stimulation, mindfulness, practice of Chinese medicine, the Ayurveda, and especially nutrition. In the latter set of actions, the use of nutraceuticals appears to be of enormous relevance and one of most effective preventive actions [6]. On the other hand, these approaches need to be accompanied by using early detection tools including molecular biomarkers. Early detection of cognitive impairment in asymptomatic patients constitutes a warning alarm to promote the use of nutraceuticals [8,9,10,11].
Functional foods are those that are considered beneficial for health and that go beyond simple nutrition: some in their natural form, such as fish or vegetables; others are preparations such as preprobiotics that are important in protecting the organism against chronic diseases and/or pathological disorders [12]. Many bioactive compounds are present in food but at low concentrations, such as flavonoid and anthocyanins in fruits and vegetables, but if used in a concentrate preparation these can be nutraceutical products that strongly contribute to the integral health of individuals [5,13,14]. Smart “drugs” quickly boost cognition. For those seeking a natural approach, four plant extracts improve brain processing speed, memory, learning, and mental concentration: blueberries, rosemary, curcuma, and garlic.
A nutraceutical is a biopharmaceutical product of natural ingredients that exhibits reliably beneficial actions in human health. This includes medicinal products made with natural ingredients. New nutritional trends and the need to meet social and health demands drove the increasing demand of functional and nutraceutical foods that, in addition to their general nutritional functions, have properties for maintaining health and longevity. One of the main challenges facing this revolution of nutraceutical foods is the absence of a single and universal definition, as well as a legal regulation of them.
2. Mechanistic Insights of Potential Nutraceuticals in AD
2.1. Quercetin/Apple
Quercetin belongs to a subcategory of flavonoids called flavanols and is one of the most consumed molecules of these compounds within the human diet, being consumed on a daily average of 5 to 40 mg [17,18]. The chemical structure of quercetin consists of three ring structures and five hydroxyl groups. It can cross the blood brain barrier, which is an important feature in the neurodegenerative disease’s context [5,19]. Quercetin has multiple properties that are beneficial for human health including anti-inflammatory and antioxidant capacities [19]. The latter is especially important in the context of neurodegenerative diseases because the brain is an organ susceptible to oxidative stress due to its high composition of unsaturated fatty acids, high oxygen consumption, and low antioxidant capacity [19].
Flavonoids are some of the major categories of antioxidants that can be found in apples, being quercetin one of the most important ones within this classification [7]. Quercetin can be extracted from the whole fruit, but apple peel contains greater amounts of this substance rather than the flesh of this fruit [7]. It was estimated that apples contain 2.1 to 7.2 mg/100 g of quercetin, which is mostly found in its glycoside form that is soluble in water [20]. All the above-mentioned effects are summarized in Figure 1.
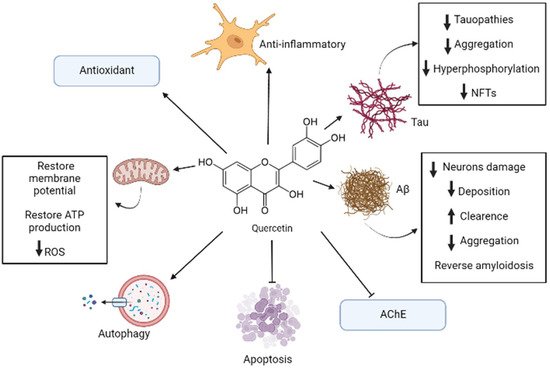
Figure 1. Representative model of anti-Alzheimer’s disease (AD) mechanisms of quercetin. In AD, quercetin induces multitargeted molecular mechanisms including antioxidant, antiapoptotic, anti-inflammatory, antitau, and anti-Aβ action. It is also involved in autophagy promotion, acetylcholinesterase (AChE) inhibition, and reversing mitochondrial disruption. The black arrow up (increase), the black arrow down (decrease). Thin arrow (stimulation) and line (Inhibition).
Some studies showed that quercetin is capable, in low concentrations from 5 to 10 μM, of reducing the damage and cell death caused by treatments with H2O2 and Aβ in cell models of PC12 cells and primary neuronal cultures, respectively [19]. Moreover, studies in the murine triple-transgenic Alzheimer’s models showed that, treatment with quercetin can significantly reverse pathological processes, such as β-amyloidosis, tauopathies, astrogliosis, and microgliosis. The test animals also improved in their memory and learning performances [19]. It was also shown in in vivo studies with rodents that quercetin’s administration (0.5 to 50 mg/kg) has a protective effect against oxidative stress and against the damage caused by various neurotoxic components [18].
In silico analysis also revealed that quercetin has a superior inhibitory capacity over AChE than that of conventional drugs used to treat AD, because quercetin (especially in its methylated form azaleatin) presents a stronger union with the active site of this enzyme as compared to that of conventional drugs used in clinical practice [19].
The antioxidant properties of quercetin are mostly given by its capacity of scavenge free radicals, its metal chelating ability, and its capacity to protect neurons against the toxicity of metals [17,18]. Quercetin can modulate enzymatic systems, such as the nitric oxide synthase, and transcriptional factors, such as NF-κβ and Nuclear factor erythroid 2-related factor 2 (Nrf-2), that induce genes that code for detoxifying and antioxidant proteins [17,18]. Quercetin can also modulate pathways that are involved in cognition, neurogenesis, and neuronal survival, such as PI3K/Akt, tyrosine kinases, Protein kinase C (PKC), and mitogen-activated protein kinase (MAPK) [17]. The activation of the Nrf2-ARE pathway has a protective effect in neurons against the damage caused by oxidative stress and against cell death; new evidence even suggests that this pathway can modulate the formation and degradation of misfolded protein aggregates present in neurodegenerative diseases such as AD [18].
Quercetin also influences the mitochondria, decreasing the dysfunction in this organelle, reducing Reactive oxygen species (ROS) production and restoring the mitochondrial membrane’s potential and production of ATP [21]. Quercetin also regulates the expression of AMP-activated protein kinase (AMPK), which has a very important role in modulating energy metabolism and reducing ROS production [21]. Another important property of the AMPK in the context of AD, is that these proteins reduce the deposition of Aβ, induce its clearance, and regulate the processing of its precursor protein APP [21].
In this context, quercetin has also an anti-inflammatory effect due to scavenging free radicals and ROS [22]. It was also demonstrated that quercetin can inhibit the expression of TNF-α at the gene expression level by modulating the activity of NF-κβ [6]. In glial cell models induced by Lipopolysaccharide (LPS), quercetin can reduce the mRNA levels of TNF-α y IL-1α, and in neurons and microglia cocultures, quercetin reduces the apoptotic neuronal death induced by microglial activation [22]. Besides, quercetin is involved in promoting autophagy, which is a very important process in maintaining the integrity of the central nervous system, and has a neuroprotective effect [18]. Quercetin can also activate SIRT1 protein which, in turn, can suppress Bax-dependent apoptosis and inhibit proapoptotic transcriptional factors [18].
In the context of AD, in vitro studies using quercetin, probed that this compound has an effective capacity inhibiting protein aggregation of Aβ, tau protein and α-synuclein by stabilizing the oligomeric forms of these misfolded proteins and through this inhibiting fibril growing [5].
Quercetin can also, due to its chemical structure and interaction with factors like BACE-1 and NF-κβ, inhibit the formation of Aβ oligomers and destabilize its fibrils, reducing the neurotoxic effects of this protein aggregates [21]. In another study, using HT22 hippocampal neurons, pretreatments with quercetin demonstrated to inhibit tau hyperphosphorylation [5]. This compound also has the capacity of inhibiting the activity of the CDK5 enzyme, a key component in the regulation of tau [5]. Furthermore, studies involving the triple-transgenic mouse models of Alzheimer’s disease showed that quercetin can reduce the levels of NFTs, Aβ and cognitive impairment in these mice [5].
Another action of quercetin has to do with the process of cellular senescence, which is defined as a permanent arrest of the cell cycle [23]. This process occurs under different conditions such as tissue remodeling in the context of development or after tissue damage. The senescence can also decrease the regenerating ability of the organic tissue and cause inflammation [23]. Cellular senescence is an important process in AD, because it can occur during aging neurons, astrocytes, and microglia that is characterized by the production of inflammatory substances and decreased functionality of tissues/organs [24]. In this context, quercetin was shown in a selective way to eliminate senescent cells in the brain of Alzheimer murine models, suggesting that quercetin has a senolytic activity [24,25].
Within the human diet, fruits and vegetables are important sources of antioxidants and other important substances that are beneficial for health [26]. Apples were proved to be a major source of antioxidants due to its high content of these kind of compounds and due to the high level of consumption of these fruit on the human population [26]. Moreover, several beneficial effects were attributed to this plant, such as anti-inflammatory, antiulcer, and neuroprotective effects [27].
2.2. Anthocyanins/Berries
In general, berries are characterized by their high content of minerals, vitamins, dietary fiber, phenolic compounds, and organic acids. However, anthocyanins (ANT) are the main bioactive compounds considered a water-soluble dye. Red wine, blueberry, bilberry, cranberry, elderberry, raspberry, strawberry, maqui, and calafate (endemic Patagonian fruit) are rich sources of natural dietary anthocyanins. Berry extracts were associated with protective effects against AD and other disorders [20,28].
Functional studies in humans associated the intake of berries with slower rates of cognitive decline in elderly subjects, suggesting the protective role of ANT on different cognitive functions [29,30]. The 30 mL blueberry supplementation (387 mg ANT) in healthy older adults showed significant increases in brain activity within areas associated with cognitive function (Brodmann areas, precuneus, anterior cingulate, and insula/thalamus) [31]. A randomized, double-blind, placebo-controlled trial, the older adults with cognitive complaints improved cognition after the long-term supplementation (24-weeks) with blueberry [32]. This shows that ANT-rich berries supplementation has neurocognitive benefit in this at-risk population for dementia [30]. However, the preventive effect will depend on the amount and ANT-structure (aglycone or its glucoside conjugated).
Anthocyanins-berries have interesting pharmacological activities, such as antioxidant and anti-inflammatory, and improve neuronal and cognitive brain performance [29,31]. Regarding the mechanism of action, it was proposed that ANT inhibit tau hyperphosphorylation and activation of GSK-3β induced by Aβ in PC12 cells [5,33]. Structurally, the planar aromatic ring of anthocyanins is essential to inhibit heparin-induced filament formation of tau protein [34]. Other studies showed that inhibition of oxidative stress and neuroinflammation are two critical mechanisms by which ANT produce protective effects in AD prevention or treatment [35] (Figure 2). Long-term, the ANT can upregulate p-PI3K, p-Akt y p-GSK-3β expression, decrease ROS and Malonaldehyde (MDA), and increase Nrf2 nuclear translocation and glutathione cysteine ligase modulatory subunit (GCLM) and HO-1 expression in the hippocampus of APP/PS1 mice [35].
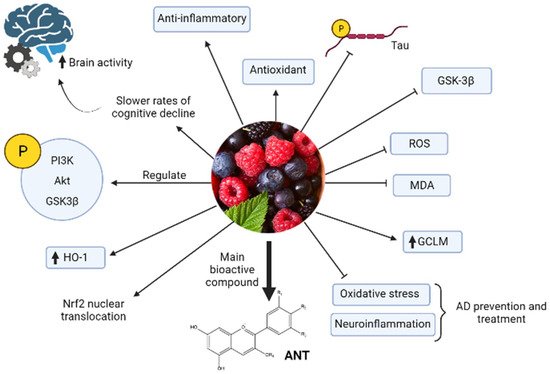
Figure 2. Model of anthocyanin properties against AD. Berries and their main bioactive compounds anthocyanins (ANT) have anti-inflammatory, antioxidant, and neuroprotective properties that make possible prevention and treatment of AD through different mechanisms. The black arrow up (increase), the black arrow down (decrease). Thin arrow (stimulation) and line (Inhibition).
2.3. Polyphenols/Honey
Honey was studied since the early 1970s, due to its nutraceutical properties that include antibacterial, bacteriostatic, anti-inflammatory, and wound and sunburn healing activities [36]. In addition to those properties, novel studies demonstrated several antioxidant and nonperoxide-dependent properties [37]. One of the main reasons behind these properties are the polyphenols present in it [38,39], which can also provide highlights regarding the honey’s botanical origins [40].
The latter is important in neurodegenerative diseases such as AD, Parkinson’s disease (PD), Huntington’s disease (HD), and multiple sclerosis (MS) [39]. In all cases, an increased oxidative stress due to the depletion of antioxidants, neuro-inflammation, prions, protein and mitochondrial dysfunction, glutamatergic excitotoxicity, and genetic alterations lead to a dysfunction or death of nerve cells [41]. Accordingly, polyphenols found in honey can prevent neurodegenerative disease in several ways [42]: (i) antioxidant effect in neurons [43]; (ii) enhancement of neuronal function and regeneration [44]; (iii) protection of neurons from Aβ-induced neurotoxicity [45]; (iv) protection of hippocampal cells against nitric oxide-induced neurotoxicity [46]; and (v) modulation of neuronal and glial cell signaling pathways [47].
One flavonoid present in honey is Luteolin. This bioactive compound shows neuroprotective activity against microglia-induced neuronal cell death and enhances the spatial working memory via prevention of microglia associated inflammation in the hippocampus of aged rats [48]. In another study, luteolin enhanced basal synaptic transmission while facilitating the induction of long-term potentiation (LTP) by high-frequency stimulation in the dental gyrus of the rat hippocampus through the activation of cAMP response element-binding protein (CREB); thus, it protects synaptic function and restores memory in neurodegenerative disorders [49]. Consistent with its neuroprotective activity, luteolin also protects against β-amyloid-induced toxicity in rat-cultured cortical neurons [50].
Other flavonoid present in honey is Kaempferol. This molecule exhibits a neuroprotective effect in Parkinson’s disease mice models induced by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The latter leads to behavioral and biochemical alterations similar to PD, such as behavioral deficits, depletion of dopamine and its metabolites, reduction in SOD and glutathione peroxidase (GSH-PX) activities and elevation of MDA levels in the substantia nigra of mice. When kaempferol was administrated to mice every 24 h for 14 consecutive days, the behavioral and biochemical alterations improved substantially. Neuroprotection was confirmed by the histochemical findings, in which kaempferol prevented the loss of TH-positive neurons induced by MPTP [51].
Another polyphenol present in honey, Ferulic acid, promotes a neuroprotective effect during a middle cerebral artery occlusion as it decreases phospho-PDK1, phospho-Akt and phospho-Bad levels, while preventing the increase in caspase-3 levels [52]. Ferulic acid also displayed a neuroprotective effect against oxidative stress associated apoptosis through inhibition of ICAM-1 mRNA expression and by decreasing the number of microglia/macrophages after cerebral ischemia/reperfusion injury in rats [53]. Also, it was demonstrated its anti-inflammatory and antioxidative properties during a transient-focal ischemia in rats [54].
On the other hand, chlorogenic acid also present in honey exerts a neuroprotective effect against methyl mercury-induced apoptosis in pheochromocytoma-12 (PC12) cell lines. In this study, chlorogenic acid prevents the generation of reactive-oxygen species (ROS), suppressing the decreasing action of glutathione peroxidase (GPx) and Glutathione (GSH) and attenuating apoptosis by the activation of caspase-3 [55] (Figure 3). It also reflects neuroprotective effects in scopolamine-induced learning and memory impairment by inhibiting the activity of acetylcholine esterase and MDA in the hippocampus as well as in the frontal cortex in mice, as demonstrated by Kwon et al. (2010) [56].
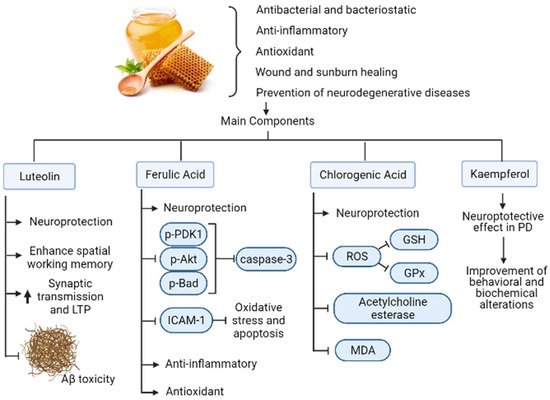
Figure 3. Mechanisms of action of honey polyphenols in AD. Honey has beneficial properties for human health that include antibacterial, anti-inflammatory, antioxidant, wound healing, and prevention of neurodegenerative diseases such as AD. Their main components consist of flavonoids and polyphenols that exert those properties as well as neuroprotective, antiapoptotic, anti-Aβ and synaptic transmission, and memory enhancement activities. The black arrow up (increase), the black arrow down (decrease). Thin arrow (stimulation) and line (Inhibition).
This entry is adapted from the peer-reviewed paper 10.3390/biom12020249
This entry is offline, you can click here to edit this entry!
