Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
The scientific community has increasingly focused on forming transformation products (TPs) from environmental organic pollutants. However, there is still a lot of discussion over how these TPs are generated and how harmful they are to living terrestrial or aquatic organisms. Potential transformation pathways, TP toxicity, and their mechanisms require more investigation.
- environmental contamination
- transformation products
- non-target screening
1. Introduction
In vitro and in vivo metabolic processes may transform organic pollutants into highly reactive metabolites. The principal biotransformation routes accompany reduction, oxidation, and/or hydrolysis during the phase I reaction. In contrast, the phase II reaction mainly features conjugation reactions. Phase II reactions are biosynthetic, as active enzymes connect the metabolite produced by phase I responses to an endogenous polar molecule, resulting in a conjugate. Many endogenous compounds with high polarity (e.g., sugar, amino acids, sulphates, etc.) are used in conjugation, and the resultant conjugates are mostly ionized and highly water soluble. Furthermore, specialized active transport systems identify the moieties employed for conjugation, assisting translocation across plasma membranes and increasing the excretion rate [107,108]. The endoplasmic reticulum, lipoprotein membranes stretching from mitochondria and nucleus to the plasma membranes of cells, are the primary sources of phase I enzymes in cells. As lipophilic substances preferentially diffuse into lipid membranes the presence of phase I enzymes in lipid membranes has crucial implications for biotransformation [109].
Phase I reactions are more typically related to the production of reactive and more hazardous metabolites; yet, phase II processes, as well as combinations of phase II and phase I processes, may be considered an intoxication procedure [110,111]. However, there is evidence that metabolites of pollutants such as tetrabromobisphenol-A, trenbolone, triclosan, and bisphenol A retain the bioactive moieties and preserve inherent toxicity comparable to the parent compound [112,113,114]. Methylation in biological systems can produce hydrophobic and bioaccumulative metabolites, often observed in fungus, plants, and bacteria [115]. Compound biotransformation studies are vital to understand the reactivity and toxicity of organisms. Bioaccumulation and toxicity of organic pollutants are heavily influenced by biotransformation, while this process is still poorly understood for emerging contaminants [116]. There have been limited investigations for TPs of EPs in specific organisms, as follows.
2. Algae
Cymbella sp. were studied for their ability to biotransform triclosan. The results demonstrated that triclosan and its potential hazardous metabolites had a high toxic impact on Cymbella sp., with 72 h EC50 of 324.9 mg/L. In diatom cells, 11 metabolites were found and with potential degradation pathways. The transformative reactions of triclosan in Cymbella sp. included methylation, hydroxylation, amino acids conjunction, dichlorination, and glucuronidation, which resulted in biologically active products (e.g., methyl triclosan) and conjugation products (e.g., or oxaloacetic acid conjugated or triclosan glucuronide) [117].
3. Freshwater Crustaceans
Biotransformation pathways in freshwater crustaceans have been little understood, except in a study using Gammarus pulex (G. pulex) and Daphnia magna (D. magna). For 24 h, G. pulex and D. magna were exposed to a modest dose of biocides and pharmaceuticals and sacrificed to identify their metabolites. Each species produced 25 and 11 metabolites, respectively, for terbutryn, irgarol, venlafaxine, and tramadol, mainly via oxidation and conjugation reactions. Affinity in the synthesis of metabolites, such as oxidation and demethylation products, were found for venlafaxine and tramadol, which have an identical backbone structure. Tramadol and venlafaxine were oxidized at the amine or cyclic C-H bond, while irgarol and terbutryn were oxidized at the terminal methyl group (MTE258B, MIR270B, MIR270A, MTE258A, and MIR286) (Figure 4) [118]. In Gammarus pulex (G. pulex) and Hyalella azteca (H. azteca), Fu et al. (2020) found a substantial pathway for diclofenac metabolism [119]. The LC–HRMS/MS data collected from the test species were used to identify metabolites utilizing NTS procedures. As a result, 281 metabolites were identified based on the isotopic signature of chlorine (Cl). H. azteca and G. pulex had nine distinct diclofenac metabolites.
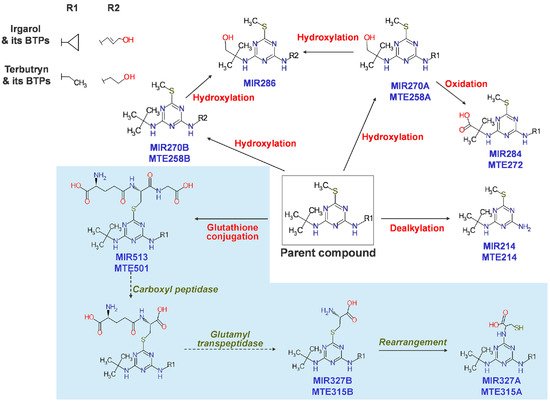
Figure 4. Proposed biotransformation pathways of irgarol and terbutryn in freshwater crustaceans. Note that R2 is the hydroxylated moiety of R1. The sky-blue shaded area indicates a pathway including glutathione conjugation followed by subsequent reactions to form cysteine conjugates, reported for the first time in the test organisms. (Reprinted from [118], Copyright 2013, ACS).
4. Fish
Metabolites of diclofenac were also identified in vertebrates. Oncorhynchus mykiss, have produced hydroxylate and conjugate of diclofenac with glucuronic acid, glutathione, and sulfate. Other EPs, including pharmaceuticals (propranolol, carbamazepine) and insecticides (diazinon, azoxystrobin, and fipronil), were found in the S9 extract of trout liver [120]. It was revealed that each of the five parents had ten distinct metabolites. The primary metabolic mechanisms were oxidation, dealkylation, S-oxidation, and epoxidation. The formation of metabolites for fipronil and diazinon was enhanced as increasing carbamazepine concentration in the binary exposure, whereas the transformation kinetic for propranolol and azoxystrobin was decreased. Toxic diazoxon and less toxic pyrimidinol, among significant diazinon metabolites, were promptly formed by S9 after the binary exposure with carbamazepine.
5. Earthworm
High-production-volume surfactants, also known as polyfluoroalkyl phosphate esters (PAPs), are employed in the packaging industry and food contact paper. PAPs can transform into perfluoroalkyl carboxylic acids, which are highly bioaccumulative and persistent in the environment, although their fate remains unknown in terrestrial species. To investigate biotransformation, Zhu et al. (2021) subjected M. guillelmi to soil contaminated with 6:2 fluorotelomer phosphate diester (6:2 diPAP). According to in vitro desorption tests [121], 6:2 diPAP desorbed from soil was considerably accumulated in gut digesting fluid. Phase I products included perfluoropentyl propanoic acid, perfluorohexanoic acid, 2-perfluorohexyl ethanoic acid, perfluoropentanoic acid, and perfluoroheptanoic acid, all of which confirmed that β and α oxidation occurred in earthworms. As a phase II product, 6:2 fluorotelomer alcohol–sulfate conjugate was found at unusually high quantities in earthworms for the first time, which may be the principal mechanism by which earthworms remove 6:2 diPAP.
6. Human Cell Lines
Using human skin subcellular fractions, the in vitro metabolism of 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and a mixture of Bis(2-ethylhexyl) tetrabromphthalate (BEH-TEBP), EH-TBB, and triphenyl phosphate was evaluated for the first time. Analysis of EH-TBB and THP utilizing UPLC-Q-Exactive Orbitrap identified the two primary metabolites, tetrabromobenzoic acid (TBBA) and diphenylphosphate (DPhP). It was assumed that CYP450 enzymes were responsible for the dermal biotransformation of TPhP and EH-TBB, but no stable metabolites were found for BEH-TEBP [122].
This entry is adapted from the peer-reviewed paper 10.3390/toxics10020054
This entry is offline, you can click here to edit this entry!
