Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Humans are exposed to a complex mix of man-made electric and magnetic fields (MFs) at many different frequencies, at home and at work. Epidemiological studies indicate that there is a positive relationship between residential/domestic and occupational exposure to extremely low frequency electromagnetic fields and some types of cancer.
- magnetic field
- cancer
1. Introduction
Public concern about electromagnetic fields (EMFs) from power systems is increasing along with the electricity demand, wireless technologies, and changes in work systems and social behavior [1,2,3,4]. For modern populations, extremely low-frequency (ELF) electric and magnetic fields (MFs) are common exposures and complex biological mechanisms underly the potential effects of externally-applied MFs [5,6]. In 2002, the International Agency for Research on Cancer (IARC) categorized ELF (including the power frequencies of 50 and 60 Hz) MFs as “possibly carcinogenic to humans” [7].
2. Epidemiological Studies Evaluating MF and Cancer Relationships
It is known that epidemiological studies alone cannot be used to determine a clear cause and effect relationship when considering MF and cancer. This is mainly because epidemiological studies evaluate only the statistical associations between exposure and disease, which may not be necessarily caused by the exposure. Only the presence of a consistent and strong association between the exposure and the effect, a clear dose-response relationship, support that is provided by relevant animal studies, a credible biological explanation, and above all, if there is consistency between the studies can support cause and effect conclusions. In studies involving EMF and cancer most of these factors are generally missing. Studies of the potential health effects of EMF have concentrated on the MF because it is generally assumed to be the component that is most likely to have biological effects [28]. Since the first evidence determining the relationship between the ELF EMF and leukemia in children [29], epidemiological studies in this context increased and the IARC classified the ELF EMF in group 2B, a “possible carcinogen” to humans, whereas static electric and MFs are not classifiable as carcinogenic to humans (Group 3) [30]. Although it is generally accepted that EMFs can exert biological effects, in general, epidemiological studies show a weak and sometimes inconsistent association between exposure to power-frequency fields (PFF) and cancer. In most cases, the studies fail to show a dose-response relationship [31,32]. The opposite happens in laboratory studies where PFF points towards causing or contributing to cancer (see below). The application of “Hill’s criteria” (i.e., strength, plausibility, specificity, biologic gradient, consistency, coherence, experimental evidence, temporality, and analogy) to laboratory and epidemiological studies shows a weak evidence for a causal association between cancer and the exposure to PFF [33].
Because cancer is one of the significant problems of global health, epidemiologic studies have faced the question of whether occupational and residential exposure to ELF EMF might be carcinogenic. There are three main explanatory hypotheses that appear in the literature: (i) the EM hypothesis, attributing EHS to EMF exposure; (ii) the cognitive hypothesis, assuming that EHS results from false beliefs in EMF harmfulness; (iii) the attributive hypothesis, considering EHS as a surviving strategy for pre-existing conditions [34]. Most of the epidemiologic studies explored the association between ELF EMFs and the susceptibility to different cancers. In the next section the residential/domestic and the occupational exposure to MF as related to cancer occurrence will be described.
2.1. Epidemiology of Residential/Domestic Exposure to MF
A survey of the literature indicates that residential exposure to EMFs is associated to an increased risk of cancers, particularly breast cancer, brain tumors, and leukemias. However, most of these studies are based on small numbers of high field-exposed cases [16,35,36] and an increasing number of studies does not support an epidemiologic association of adult cancers with residential MFs [35,36,37]. Many of the studies clearly have shortcomings, which often prevent any firm conclusions [38,39]. Moreover, the indirect measures of EMF exposure used may also correlate with other factors such as social status (e.g., age, race, gender) or environmental pollution. It is possible that these unconsidered and confounding factors may contribute to the cancer rates that are reported and also to the contrasting results that are reported in various EMF studies [40]. Indeed, EMF, which itself is not believed to be genotoxic, could influence carcinogenesis if it exerted either direct or indirect effects on target cells [41].
Another important issue is the exposure assessment. The exposure to MF can vary greatly over time and distance, has multiple sources, and is imperceptible and ubiquitous [42,43,44,45]. In exposed schools, children may experience a higher chance of receiving a mean exposure >0.4 µT during school hours [46,47]; whereas those living in big buildings or using electric heating appliances in larger families had a generally higher level of personal indoor exposure [48]. Based on the known location of domestic and service MF sources, apartments can be reliably classified as high and low MF-exposed [49,50,51]. Methodologies for estimating MF at study residences as well as for characterizing the sources of uncertainty in these estimates have been developed [52]. In residential/domestic epidemiological studies, geographic information that is collected in an exact place of residence at the time of cancer diagnosis can provide several strategic geophysical elements for assessment [53]. The estimation of the overall exposure level from a single address is also informative [54]. In general, the public health impact of residential fields is considered limited, but the available data show the occurrence of both no impact and substantial impact possibilities [55].
2.1.1. Brain Tumor
The incidence of primary brain tumors has increased in many countries worldwide [56,57] and gliomas are the most frequent primary brain tumors in adults [58]. Residential exposure during childhood to EMF produced inconsistent results and a lack of an association when related to brain cancers, regardless of the exposure metrics that were used whether based on wire codes, distance, or the measured or calculated fields [59]. When focusing on the health effects, the most studied sources of ELF MF are power lines. Exposure to ELF MF that is emitted by power lines can be assessed by direct methods that rely on measurements at a given place over a time range [60] or by individual monitoring through measuring ELF MF exposures throughout the day by wearable dosimeters [61]. Both methods give little or no information on historical exposure to ELF MF. Indirect methods include geographical information system (GIS) which have been used along with declarative data, such as residential history, to assess residential ELF MF exposure in the general population [62,63,64,65]. Case-control studies that are based on death certificates revealed an association between adult brain tumor mortality and living less than 50 m (odd ratios, OR 1.10 95%, CI 0.74–1.64) [66] or 100 m (OR 2.99, 95%, CI 0.86–10.40) [67] from power lines. In a recent work, significant associations were found between the cumulated duration living at <50 m to high voltage lines (50/60 Hz, 0.3 μT) and all brain tumors (OR 2.94; 95%CI 1.28–6.75) and glioma (OR 4.96; 95%CI 1.56–15.77) [65], confirming previous studies [35,66,67,68,69]. Contrasting results have been reported for brain tumors in children. In childhood brain cancer, with the exception of the possibility of a moderate risk increase in high cut-point analyses (0.3/0.4 µT), no increased risk was evident for different exposure metrics [70,71], whereas children whose MF exposure level was above 0.3 or 0.4 μT, an elevated risk of brain tumor was observed [72,73,74]. In residential areas, the transient electric and MFs would induce higher current density in the child‘s body than power frequency fields with similar field strength [75]. In some studies, there is no evidence for a role of ELF cellphone EMFs in childhood brain cancer [27].
2.1.2. Breast Cancer
Breast cancer threatens women with the highest incidence and second highest mortality rate of all cancers and in women aged 65 and older when nearly one half of all new breast cancer is diagnosed [76]. The excessive exposure to MFs increases the risk of female breast cancer, as demonstrated in several pooled or meta-analyses as well as subsequent peer-reviewed studies [69]. It is questionable whether chronic human exposure to MFs might affect melatonin secretion, its circadian rhythm, or both [77,78,79,80]. In general, no cumulative effects on melatonin secretion in humans have been found in response to MFs and this rebuts the “melatonin hypothesis” in which a decrease in plasma melatonin concentration (or a disruption in its secretion) would be correlated with the occurrence of breast cancers as a consequence of exposure to MFs [81,82,83,84,85,86,87,88]. Indeed, MF exposure correlates with an increased proliferative activity of the mammary epithelium, which is a likely explanation for the cocarcinogenic or tumor-promoting effects of MF exposure that is observed [89]. However, some authors found a motivation for going back to the melatonin hypothesis in relation to data, suggesting magnetosensory disruption by ELF MF in mammals, and magnetosensitivity in humans, along with the influence of MFs on circadian rhythmicity with a consequent disruption of non-photic sensory stimuli of various nature [90].
In studies that were based on the measurement of an electric bedding device, only premenopausal breast cancer (OR = 1.23; 95% Cl: 1.01, 1.49, p = 0.04) showed a slight increased risk [91]. Breast cancer was found to increase with the number of years and seasons of bedding device use during sleep. Similar trends in dose response were shown in both premenopausal and postmenopausal women and for both estrogen receptor-positive and estrogen receptor-negative tumors. Therefore, there is a growing body of evidence that the use of electric bedding devices may increase breast cancer risk [92]. In terms of geographic variation of breast cancer rates, the results are inconclusive and do not support a major role of MF risk factors in the etiology of breast cancer [93].
2.1.3. Leukemias
Leukemia is the most common cancer in children [94]. The analysis of reports on childhood leukemia as related to exposure to MFs shows that a statistically significant association between MF exposure and childhood leukemia is found in almost all government or independent studies with an elevated risk of at least OR = 1.5, while a not significant or even suggestive association is reported in many industry supported studies [69].
Several meta-analyses showed a statistical association between childhood leukemia and a range of exposure 0.1–2.36 µT MF intensity [74,95,96,97,98,99]. Children that are exposed to elevated ELF-MF show relative risks of leukemia between 1.3 and 2 [100,101,102] and the highest exposure was associated with an increased risk of B-lineage acute lymphoblastic leukemia (B-ALL) when compared with lower exposures [103]. A significant association was observed during the night (OR = 3.21, 95% CI 1.33–7.80) between childhood leukemia and MF exposure [104].
Several epidemiologic case-control studies examined the association between childhood cancer risk and distance to high-voltage overhead transmission lines (HVOTL). Statewide, record-based case-control studies of childhood leukemia evidenced the occurrence of risk that was associated with greater exposure to MF that was generated in areas that were close to power lines [66,105,106,107,108]. Living in polluted regions and pre- and post-natal exposure to high voltage power lines has been described as risk factors of acute lymphoblastic leukemia (ALL) in people of low socioeconomic status Iranian population [109]. In children with ALL, ELF MF-exposure was found to have no impact on the survival probability or risk of relapse [110].
The occurrence of childhood acute leukemia (AL) has been studied around nuclear power plants (NPP). The results suggest a possible excess risk of AL in the close vicinity to NPP [111]. The small, but statistically significant increased incidence of AL in the surrounding of some NPP have motivated governments to work toward a better understanding of the main causes of AL long-term strategic research agendas through interdisciplinary and international efforts [112].
MFs are not the only factor that varies in the vicinity of MF sources, complicating interpretation of any associations. Several reports demonstrate that MFs that are generated by different sources are not an important cause of leukemia both in adults [37,113,114] and children in many geographical areas [12,113,114,115,116,117,118,119,120]. Moreover, exposure levels in some big cities are always significantly far lower than 0.3–0.4 µT [121].
The associations that were observed between power lines exposure and childhood leukemia appears to be not related to mobility [122,123]. No indications were also found of an association of risk for people that are exposed to magnetic fields from underground [124] and above ground lines [106,107] or of a trend in risk with increasing MF for leukemia. Residential proximity to transformer stations has been associated with a borderline risk of childhood cancer [125].
Distance from HVOTLs during the year of birth is unlikely to be associated with an increased risk of leukemia [73] and little evidence was found between exposure to MF inside infant incubators and increased risk of childhood leukemia [126]. No statistically significant association was observed between wire codes and childhood leukemia [127]. Recently, a synoptic analysis provided evidence that the risk of childhood leukemia is not increased by exposure to ELF EMFs, suggesting that IARC’s classification of ELF EMF needs revision [128].
By considering both significant and not significant correlations between MF and leukemia, no major environmental risk factors (including MFs) have been established as major contributors to the global leukemia burden, although distinct incidence patterns by geography, age, and sex suggest a role of the environment in its etiology [129]. The results may be affected by several sources of bias: analyses that are based on continuous exposure show no exposure-disease association, while incoherent exposure-outcome relationships characterize analyses that are based on categorical variables [130].
2.1.4. Other Cancers
EMF in the home-environment (color tv, computer monitor, microwave-oven, cellular phone, etc.) might act as potential contributing factors for the development of cancer, as well as exert indirect effects on humans. Microwave exposure induces L-amino acids change to D-amino acids, and exposure of the human body to microwaves over a long period of time may contribute to induction of cancer [131]. Exposure to EMF that is generated by electric blankets has been suggested to increase the risk of hormone-dependent cancers such as testicular [132] and prostatic [133], but was not significantly associated with endometrial cancer risk [134]. MFs of industrial frequency have been shown to be a risk factor for occurrence of oncological diseases in the population and an increased incidence of malignant tumors has been noted as the induction of the magnetic field that is produced by HVOTL increases [135]. The incidence of melanoma has been linked to the distance to FM broadcasting towers. A correlation between melanoma incidence and the number of locally receivable FM transmitters was found when geographic differences in melanoma incidences were compared with the magnitude of this environmental stress. Therefore, melanoma might be associated with exposure to FM broadcasting [136]. By considering pregnancy duration, neonatal birth weight, length, head circumference, gender, and con-genital malformations, no significant effects of ELF EMFs were observed; however, precautionary measures are necessary to reduce exposure to EMFs by pregnant women [137].
The Supplementary Table S1 reports the relationship between MFs and cancer in epidemiologic studies that are related to domestic/residential exposure to MFs. The type of cancer, study design, source of MF, range of MF exposure, location of the epidemiologic study, and the main conclusions are reported. Figure 2 summarizes the epidemiology of residential/domestic exposure to MF.
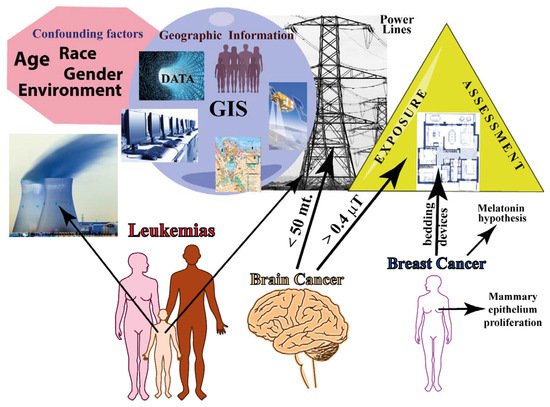
Figure 2. Summary of epidemiology of residential/domestic exposure to MF. The major confounding factors in epidemiological studies are shown along with the main geographical information that is based on GIS (data management, hardware/software, topography, remote detection, and population demographics). Exposure assessment needs to be evaluated both outside and inside the residence. The three major cancers are represented: leukemia affects mainly childhood; brain cancer increases with decreasing distances from EMF sources; and breast cancer is associated with mammary epithelium proliferation and with exposure to bedding devices.
2.2. Epidemiology of Occupational Exposure to MF
During the last few decades, the intensity level of the EM occupational environment has dramatically increased. By job category, the most highly exposed jobs (>0.23 µT) included electronics workers, cooks and kitchen workers, cashiers, bakery workers, textile machine operators, and residential and industrial sewing machine operators [138]. The main components of EM pollution are in the ELF (10–300 Hz) and in ultra-low (ULF: 0–10 Hz) frequency bands [139]. Occupational epidemiology reveals that exposure to ELF EMF is generally greater than that in the general population and concerns a large number of workers in a variety of industries (see [140,141] for a historical overview of the occupational EMF epidemiology).
MF exposure limits are more than a thousand times higher than the magnitudes that are associated with the cancer risks that are observed in epidemiologic studies, leaving millions of workers exposed to MF in this large gray area where the public health consequences are unclear [140,141]. The International Labor Organization defines occupational exposure as “exposure of a worker received or committed during a period of work” [142] while the ICNIRP defines occupational exposure as “all exposure to EMF experienced by individuals as a result of performing their regular or assigned job activities” [143].
As discussed for residential/domestic exposure to EMF, in the case of occupational exposure, contrasting results have also been presented in relation to the co-occurrence of different cancers including brain and breast cancer and hematological malignancies.
2.2.1. Brain Cancer
Occupational exposure to ELF EMF is a suspected risk factor for brain tumors; however, the literature reports contrasting results. In adults, some meta-analyses of occupational studies indicate a slightly higher risk for electrical workers, suggesting a small increase in brain cancer risk [61,144,145], including childhood brain tumors [146], while others found no evidence to support the hypothesis that exposure to MFs is a risk factor for gliomas or meningiomas [147,148,149,150].
2.2.2. Breast Cancer
Several studies have evaluated the evidence linking women’s occupation and workplace exposures to breast cancer. Overall, the data do not suggest that occupational exposures to EMF increases the risk of breast cancer [144,145,151,152] with some exceptions [153]. Some studies have found an effect when looking at overall risk elevations in the women studied [154] with increased risk among postmenopausal women but not premenopausal women [155]. The hypothesis that daytime occupational exposure to MF enhances the effects of nighttime light exposure on melatonin production (see above the “melatonin hypothesis”) has been provided [153]. Occupational MF exposure has also been suggested as a risk factor for breast cancer in men. An elevated risk of breast cancer was found in men who were exposed to 0.6 T when compared to those with exposures <0.3 T; however, large case-control studies of breast cancer in men that have been conducted to date provide limited support for the hypothesis that exposure to MF increases the risk breast cancer in men [156].
2.2.3. Leukemias
Epidemiological studies addressing occupational ELF MF exposure and risk of leukemia in adults have yielded slightly increased risks in exposed workers. Some studies show a positive correlation between occupational MF and leukemias [157,158,159,160] and have suggested that stronger effects may be observed for acute myeloid leukemia (AML) [161,162], chronic myeloid leukemia (CML), ALL [138], lymphatic leukemia (LL) [163], and for chronic lymphocytic leukemia (CLL) [162]. In general, however, no clear exposure-response pattern has emerged from the studies that evaluated exposure levels and some results do not support an association between occupational ELF MF or electric shock exposure and AML [164].
Differences between the study designs or the populations that were studied might be the cause of lack of consistency regarding the type of leukemia that is associated with MF exposure, and still no firm conclusions can generally be drawn based on the existing evidence [165,166,167]. No association was found between childhood leukemia risk and parental occupational exposure to ELF EMF [168,169,170].
2.2.4. Other Cancers
In men, exposure to EMF showed an increased incidence of tumors of the liver, biliary passages, kidney, and pituitary gland; for these cancer sites an exposure-response relation was indicated [171]. There was very limited evidence for associations between occupational ionizing radiation and testicular cancer, while there were some positive associations for EMF [172].
Women that were exposed to MF showed an increased incidence of astrocytoma I-IV, for cancer of the corpus uteri and multiple myeloma, with a clear exposure-response pattern [171].
For both men and women, there was weak support for the hypothesis that occupational MFs exposure increased the risk of non-Hodgkin lymphoma [173], acoustic neuroma [174,175], and thyroid cancer [176], while sources that produce ELF fields were not associated with neuroblastoma in offspring [177].
MF has previously been associated with mortality from acute myocardial infarction (AMI) and arrhythmia but not from chronic coronary heart disease (CCHD) or atherosclerosis in electric utility workers. For cumulative exposure, no association was observed with mortality from AMI or CCHD, indicating no exposure-related risk increase for AMI mortality, which does not confirm previous results [178].
Supplementary Table S2 reports the relationship between MFs and cancer in epidemiologic studies that are related to the occupational exposure to MFs. The type of cancer, study design, source of MF and occupation, range of MF exposure, location of the epidemiological study, and the main conclusions are reported. Figure 3 summarizes the epidemiology of occupational exposure to MF.
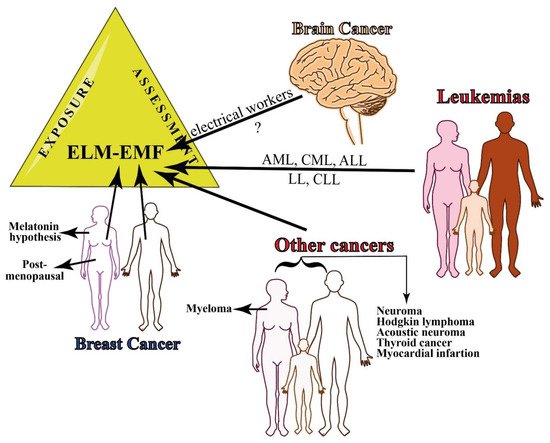
Figure 3. Summary of epidemiology of occupational exposure to MF. There is still little evidence on the relationship between occupational exposure to EMF and brain cancer, whereas several leukemias have been associated with continuous exposure to ELF-EMFs. Breast cancer occurs both in women and men and the risk increases in men that are exposed to 0.6 T. Other cancers that are associated with MF exposure include myeloma in women and several other types of cancer in both women and men.
3. In Vivo and In Vitro Effects of Magnetic Fields on Cancer
In many studies ELF exposure causes significant changes in cell survival, cell cycle progression, DNA integrity, and proliferation [40,179]. The cellular response to ELF MF may depend on many parameters including osmolarity, frequency, waveform, the strength and the exposure duration of the electromagnetic field, genetic/biological characteristics of the cells [180], specific metabolic state, or the specific stage in the cell cycle [180,181]. On the other hand, a common effect of exposure to SMF is the promotion of apoptosis and mitosis, but not of necrosis or modifications of the cell shape [182,183]. The unbalance of the apoptotic process could be linked to Ca2+ fluxes that are, in turn, dependent on the effect on the plasma membrane that is exerted by SMF [184,185,186,187]. Other possible effects of SMFs that may lead to perturbation of the apoptotic rate, such as an alteration of the gene pattern expression or the increase of oxygen free-radicals, could be, in turn, a co-carcinogenic factor leading normal cells, most likely with other sub-lethal changes, to contribute to the development of diseases [183].
The reduction of cell proliferation due to MF has been attributed to the interference of signal transduction processes due to the tangential currents that are induced around the cells [188]. The poor reproducibility might be caused by the period-dependent converse cell growth due to the MF and might explain the adverse effects that are observed in several experimental investigations [189].
Quantitative analyses of protein kinases C (PKC) expression patterns demonstrated the translocation of PKC from the cytosolic to the membrane fraction was not affected by MFs [190]. The phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) is increased in response to ELF MF; however, the small increase in ERK1/2 phosphorylation is probably insufficient to affect proliferation and oncogenic transformation [191]. Furthermore, repeated ELF EMF exposures did not show consistent response profiles to time courses of immediate early genes, apoptotic genes, cell proliferation, and stress response [192].
Small changes in transcription may occur in response to MFs [193,194]. Exposure to 900 MHz identified a differential expression of functional pathways genes [195]. Indeed, epigenetic changes, including modifications of histones and microRNA expression and DNA methylation, can be associated to ELF MF exposure [196,197,198]. However, in HeLa cells, RNA polymerase-catalyzed RNA synthesis as well as DNA polymerase-catalyzed DNA synthesis were found to not be statistically significantly affected by 60 Hz 0.25–0.5 T exposure for 0–60 min [199].
Aberrantly-expressed serum exosomal miRNAs upon MF exposure suggests a series of informative markers to identify the exact dose of MF exposure [200]. ELF MF exposure stabilizes active chromatin, particularly during the transition from a repressive to an active state during cell differentiation [201]. Membrane receptors could be one of the most important targets where ELF MF interacts with the cells [202] and RAS proteins, a member of a large family of GTP-binding proteins that are involved in intracellular signal pathways, may participate in the signal transduction process of 50 Hz MF [203]. EMF was also found to affect the proteasome functionality, inducing an increase in its proteolytic activity [204].
To answer how MF may cause cancer, the action of MF as mutagenic agents and MF involvement on chemical reactions that generate free-radicals have been considered. MF does not seem to exert mutagenic effects and the generation of free-radicals that might be linked to several other factors, beside the variability of MF exposure [40]. In the next part of this section, the current data that are related to studies on human in vitro and cell-free systems as well as in animal models will be discussed. This section will also discuss the role of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and the role of radical pair (RP) formation in MF-cancer relationship.
3.1. Studies on Human Cells (In Vitro Cellular Studies and Cell-Free Systems)
Exposure to ELF MFs combined with ionizing radiation do not suggest any synergistic or antagonistic effects on human blood cells [205], whereas, by using sister chromatid exchanges and comet assays, a slight but significant decrease of cell proliferation was evident in blood cells that were exposed to 980–1020 µT [206].
Dermal fibroblasts that were exposed to 1 mT from one to five hours showed a reduction of their viability [207], while the rePETitive exposure to MF with ELF induced DNA double-strand breaks and apoptosis in lung fibroblasts [208]. However, a longer exposure (48–72 h) to a reduced MF intensity (20–500 µT) did not result in any appreciable effect in the structural morphology and proliferation of human fibroblasts [209].
Exposure of glioma cells to MF of 1.2 µT intensity for three hours revealed changes in both gene and protein expression. Microarray results showed an up-regulation of five genes, whereas 25 genes were down-regulated upon MF exposure, suggesting a response in the glioma cells to the MF treatment [210]. Proteomic studies on glioma SF767 cells show a cytoskeletal intermediate filament protein increased following a low-level MF [211]. On the other hand no mechanisms that would explain the reported association between MF and carcinogenesis were observed in H4 glioma cells [212].
In human keratinocytes, ELF EMF induces a slight oxidative stress that does not overwhelm the metabolic capacity of the cells or have a cytotoxic effect when the cells are exposed to 25–200 µT MF intensity [213] but it does not affect melanin synthesis or skin pigmentation [214].
The adherence of leucocytes and T-lymphocytes that were taken from cancer patients strongly increased following exposure to sinusoidal MF at 50 Hz and 0.5–10 mT MF intensity for one hour [215,216], whereas a low-frequency pulsing electromagnetic field of 45 mT for three hours induces cell death in native peripheral blood mononuclear proliferating cells that were isolated from AML patients [217].
ELF MF increases the rate of cell death in normal human lymphoblastoid cell lines and is ineffective on genetic instability syndromes cell lines, i.e., Fanconi anemia group C and ataxia telangectasia, suggesting that the response of cells to ELF MF is modulated by genes that are implicated in genetic instability syndromes [218]. Exposure to a pulsating MF 50 Hz, 45 mT three times for three hours was found to protect U937 human lymphoid cell lines against puromycin-induced cell death in a cell density-dependent manner (an increased density induced cells death and prevented puromycin-induced cell death) [219].
The effects of 6- and 10-T SMFs was studied on HL-60 cells to evaluate the expression of protooncogenes. It was found that exposure to a strong MF gradient induced c-Jun expression, whereas no alteration of the expression of the c-fos and c-myc protooncogenes was observed [220]. In the same cell line, exposure to LF EMF to lower intensity (5, 300, and 500 µT) did not affect calcium signaling [221].
An ELF magnetic field was also found to influence the early development of mesenchymal stem cells that were exposed for 23 days to 50 Hz and 20 mT intensity [222], whereas no variations in surface morphology and cell death occurred between the control and the exposed osteosarcoma human cells that were exposed to 50 Hz and 0.5 mT, although significant changes were noted in cell growth [223].
Supplementary Table S3 reports the effects of MFs on human cell lines. The type of cell, response to MF, range and duration of MF exposure, materials and methods that were used, and the main conclusions are reported.
3.2. Studies on Animals
Exposure to MF was found to be a cancer promoting factor in animal experiments; animal studies are often used in the evaluation of suspected human carcinogens [224]. However, discrepancies in results were found to be associated with the use of different sub-strains, different sources for diet, environmental conditions, and MF exposure metrics [225,226,227]. The continuous monitoring of both MF and other environmental parameters is, therefore, an important part in the overall quality of the obtained results [228]. Experiments with animals were also important to determine which genetic and environmental factors are critical for potential carcinogenic or cocarcinogenic effects of ELF EMF exposure [229]. Furthermore, the genetic background was found to play a pivotal role in effects of MF exposure [230]. To study the effects of MFs on cancer cell growth, multiple exposure levels have been performed using mice and rats as experimental models.
3.2.1. Studies on Mice
Several studies revealed that there were not significant effects of MF on cancer in mouse studies, failing to support the hypothesis that acute MF exposure causes DNA damage. The experimental mice were injected with mammary murine adenocarcinoma to investigate the interaction between a 50 Hz, 2 mT MF exposure and cell growth.
Neither the time of tumor cell injection nor the time of exposure produced differences between the unexposed, sham, and exposed mice [231] and no association between exposure and the incidence of benign or malignant tumors was found in squamous cell carcinomas of mice that were exposed to 60 Hz, 2 mT [232]. Also, the results do not support the hypothesis that acute MF exposure causes DNA damage and apoptosis in the cerebellums of immature mice that were exposed to 60 Hz and 1 mT for 2 h [233,234].
Exposure to 50 Hz and 500 µT MF did not alter responses of inflammatory genes and the activation of splenic lymphocytes in mice [235] and was not a significant risk factor for hematopoietic diseases, even when high exposure levels were used (50 Hz, 1 mT for 7 days) [236]. In mice, as revealed in humans (see above), long-term and continuous exposure to simulated powerline MFs (0.5–77 µT) did not result in a decreased nocturnal melatonin secretion [85].
The use of NI MFs has shown early promise in a number of animal studies as an effective tool against many types of cancer (see also below). The effect of varying durations of MF exposure on tumor growth and viability has been studied in mice that were injected with breast cancer cells by using an in vivo imaging system. The results showed the potential of MF in cancer therapeutics, either as adjunct or primary therapy [237].
Long-term exposure showed a significant effect of MF on mice. When a parental generation of six week-old OF1 mice was exposed to an artificial ELF MF (50 Hz, 15 µT) for 14 weeks, activated partial thromboplastin time and reptilase time values significantly increased in the female mice, which also showed a very significant shortening of the prothrombin time that was associated with ELF MF exposure [238]. A chronic exposure of mice to a 60 Hz and 110 µT intensity was found to influence some hematologic parameters and the weight of thee liver and also caused spleen hyperfunction [239]. Long-term exposure to MF (50 Hz, 50 µT) was found to be a significant risk factor for neoplastic development and fertility in C57BL/6NJ female mice and C3H/HeNJ male mice [240].
3.2.2. Studies on Rat
Both positive and negative effects of MF have been demonstrated in studies using rats. Artificial MFs of 1 mT that was administered for seven weeks was not carcinogenic nor cancer-promoting for colon carcinogenesis in male Sprague–Dawley rats [241]. No overall effects of 60 Hz and 1 mT MF on splenomegaly or survival were found in the exposed Fischer/344 rats. In addition, no significant and/or consistent differences were detected in the hematological parameters between the exposed and control rats [242]. MF exposure for 14 weeks to 6.45 µT did not appear to be a strong co-tumorigenic agent in Sprague–Dawley female rats mammary, lung, and skin models [243]. On the other hand, EMFs resulted in significant alterations in cell adhesion mechanisms when histological, immunohistochemical, and histopathological analyses were performed on Wistar albino rats that were exposed for six months to 5 mT MFs [244]. MF exposure (50 Hz, 100 µT for 2–26 weeks) of female Sprague–Dawley rats resulted in an increased proliferative activity of the mammary epithelium which was associated with the cocarcinogenic or tumor-promoting effects of MF exposure that was observed in the 7,12-dimethylbenz(a)anthracene model of breast cancer [89], and mammary tumorigenesis [245].
The Supplementary Table S4 reports the effects of MFs on both mice and rats. The type of cell, response to MF, range and duration of MF exposure, materials and methods used, and the main conclusions are reported. Figure 4 summarizes the in vivo and in vitro effects of magnetic fields on cancer from studies on human cells (including cell-free systems) and in animals (mice and rat).
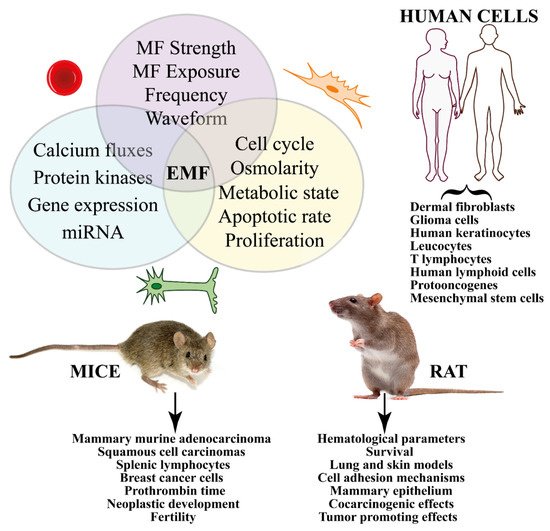
Figure 4. Summary of the in vivo and in vitro effects of MFs on cancer from studies on human and animal cells. Different treatments (e.g., strength, duration, frequency, etc.) induce signal transduction pathways that eventually trigger gene expression. In vitro studies show significant effects on cell cycle, proliferation, and apoptosis. Human cells have been used to evaluate the effect of MF on several cancer types, whereas animal experimentation has been focused on mice and rats.
3.3. Involvement of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS)
ROS (such as superoxide [O2•−] and hydrogen peroxide [H2O2]) and RNS (e.g., nitric oxide [NO•]) are generated during the oxidative cell metabolism. The cellular oxidative stress depends on the balance between the production of ROS and RNS and the activity of the antioxidant system. Excessive ROS/RNS, which is caused by the deregulated redox homeostasis, is a hallmark of disease [246]. Free-radical-scavenging enzymes, such as catalase (CAT), glutathione peroxidase (GPX), and superoxide dismutase (SOD), are the first line of defense against oxidative injury [247]. After EMF exposure, significant variations in the total antioxidant activity; vitamin E and vitamin A concentrations; increase of malondialdehyde (MDA, a product of polyunsaturated fatty acid peroxidation which is used as an indicator of oxidative stress in cells and tissues); and plasma selenium concentration in erythrocytes have been observed [248]. A recent review concluded that most animal and many cell studies show increased oxidative stress that is caused by MF [249]. Free-radical formation and the consequences of their effects on living systems explains the increased cancer risks that are associated with mobile phone use, occupational exposure to MF, and residential exposure to power lines [250].
ROS may be involved in RP reactions that have been considered as one of the mechanisms of transduction that is able to initiate EMF-induced oxidative stress [19]. It is known that applied MFs and magnetic isotope substitution can alter the rates and product yields of free-radical reactions with the formation of transient paramagnetic intermediates in non-equilibrium electron spin states. The most common source of spin-chemical effects are organic RPs. Typically formed in a singlet (S) or a triplet (T) state by a reaction that conserves electron spin, RPs interconvert coherently between their S and T states as a result of the Zeeman, hyperfine, exchange, and dipolar interactions of the electrons and the nuclear spins to which they are coupled [251]. Applied MFs alter the extent and timing of the S⇆T interchange and hence the yields of products that are formed spin-selectively from the S and T states [252]. MFs and spin effects have proven to be useful mechanistic tools for radical mechanism in biology [252] and the RP mechanism (RPM) has been associated with increased levels of ROS [253]. Moreover, the role of cryptochromes as the putative magnetosensitive molecules in magnetoreception has been considered in the RPM and discussed as related to cancer-relevant biological processes [254]. The results are also consistent with MF effects on light-independent radical reactions [255].
Significant increases in ROS levels have been found to influence the hepatic redox state [256] and were observed in several cell lines just after the end of ELF EMF exposure [213,257,258,259,260,261,262,263,264,265]. ELF EMF exposure also elevates the expression of RNS and O2•−, which are countered by compensatory changes in antioxidant CAT activity and enzymatic kinetic parameters that are related to cytochrome P450 (CYP-450) and CAT activity [266]. Moreover, the modulation in kinetic parameters of CAT, CYP-450, SOD, and MDA concentration and iNOS enzymes in response to ELF EMF [267,268,269] and the negligible effects on GPX [270] indicates an interaction between the ELF EMF and the enzymological system. SMF promoted the release of ferrous iron (Fe2+) and induced the production of ROS in osteosarcoma stem cells [271]. Superoxide increased in the micronucleus and mitochondria with an exposure-response relationship and cytosolic superoxide increased at 10 µT in SH-SY5Y and C6 cells [272]. These results confirm that the threshold for biological effects of ELF MFs may be as low as 10 µT.
In liver tissue of female rats, long-term ELF-MF exposure enhanced the oxidative/nitrosative stress and might have a deteriorative effect on cellular proteins rather than lipids by enhancing 3-nitrotyrosine formation [273]. MF appears to induce apoptosis effects through oxidative stress [274] and mitochondrial depolarization [275], whereas the influence of correlations between ELF EMF and vitamin E supplementation have been shown on antioxidant enzyme activity in AT478 malignant cells in vitro [276]. In embryonic stem cell-derived embryoid bodies, exposure to 10 mT MFs increased ROS [277].
Supplementary Table S5 reports the effects of MFs on ROS and RNS. The type of cell/tissue/organ, response to MF, range and duration of MF exposure, materials and methods used, and the main conclusions are reported. Figure 5 summarizes the involvement of ROS and RNS in the cellular and organismic responses to MFs.
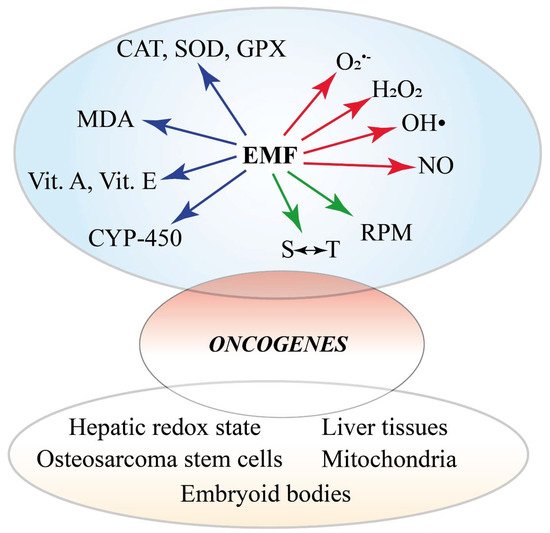
Figure 5. Summary of the involvement of ROS and RNS in the cellular and organismic responses to MFs. There are three major effects of varying MF the are reported. MFs alter the redox status of the cell by affecting the activity and gene expression of ROS-scavenging systems, including CAT, SOD, GPX, vitamins, and monooxygenases. Membrane degradation is evidenced by MDA detection. On the other hand, MFs trigger the production of ROS and NOS and early events involving the radical pair mechanism and spin-chemical effects. The altered oxidative status eventually induces the expression of oncogenes. The alteration of the oxidative status is also evident at the subcellular, cellular, tissue, and organ level.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031339
This entry is offline, you can click here to edit this entry!
