Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The term algae encompasses a wide range of photosynthetic organisms that are found primarily in freshwater and marine environments, although certain representatives can thrive in terrestrial niches, either on their own or by developing symbiotic relationships with other organisms.
- cyanobacteria
- algal extracts
- antimicrobial
1. Introduction
The term algae encompasses a wide range of photosynthetic organisms that are found primarily in freshwater and marine environments, although certain representatives can thrive in terrestrial niches, either on their own or by developing symbiotic relationships with other organisms [1,2]. Larger eukaryotic algae, such as seaweeds, are called macroalgae, while smaller unicellular eukaryotes are collectively referred to as microalgae. On the other hand, cyanobacteria (blue-green algae) represent the prokaryotic clade of this highly diverse group [2]. Moreover, some genera exhibit extraordinary growth potential in terms of produced biomass within a short period of time [3,4].
Due to the high biomass production potential and their ability to grow relatively quickly, recent research on algae has been primarily focused on biofuel production. They are more promising alternatives than plant-based biofuel production, because, unlike plants, they do not require fertile land for growth; thus, they do not interfere with food production [2,5,6]. However, significant research effort has been made in recent years in identifying and isolating bioactive compounds from algae. These compounds may possess pharmaceutical or biomedical value and can be used in anticoagulant, antioxidant, antitumor, antimicrobial, immunomodulatory, antilipidemic, hypoglycemic, and anti-inflammatory products [7,8,9,10]. Algae and their metabolites can also be used in cosmetics due to their antioxidant or tissue regenerative action and in the food industry, due to their high content of fibers, minerals, vitamins, pigments, and antioxidants [8]. In terms of biochemical properties, these metabolites can be lipids, proteins, peptides, polysaccharides, carotenoids, phenolics, and alkaloids [5,11]. Such bioactive compounds can be produced on an industrial and agricultural scale, providing substantial economic benefits [8]. Furthermore, recent advancements in microalgae biotechnology have made it possible to use microalgae as bioreactors for recovering recombinant proteins and medicinal products such as vaccines, antibodies, immunotoxins, and antimicrobial agents [3,12].
Algae and their extracts also display antimicrobial, nematocidal, herbicidal, and insecticidal/acaricidal properties against crop pathogens and can be used as biopesticides [13,14,15]. The term biopesticide may encompass naturally occurring compounds (e.g., sodium bicarbonate, sodium acetate), biochemical substances that are produced naturally by various organisms or by genetically-modified plants, as well as microorganisms (bacteria, viruses, fungi, algae etc.) that are used to control pests in agricultural practices. In this regard, algal components have been shown to efficiently suppress plant pathogenic bacteria, including the genera Agrobacterium, Pseudomonas, Xanthomonas, and Erwinia, which are associated with serious diseases of important crops that form the nutritional basis of most human societies, such as rice and potato plants [16,17,18]. Similar effects have been identified against the most common plant pathogens, fungi. Algal extracts can inhibit mycelial growth and induce resistance in plants against widespread fungal genera such as Fusarium, Verticillium, Rhizoctonia, Phytophthora, and Phoma [18,19,20]. Their antimicrobial activity also expands to viral pathogens, such as tobacco mosaic virus (TMV) and potato virus X (PVX), which are inhibited either directly or by inducing plant defense mechanisms [21,22,23]. As well as effectively controlling pathogenic microorganisms, algal extracts have been tested with success against animal targets. These targets include soil-borne nematodes, plant or fruit feeding insects (e.g., fruit fly larvae) and mites, as well as insects that mediate transmission of diseases [24,25,26,27]. Herbaceous weeds that hinder crop development, as well as algal species that grow uncontrollably resulting in harmful algal blooms, can also be treated with algal products. The effect in these cases, are either based on cytotoxicity or the inhibition of photosynthesis [28,29].
2. Biological Roles of Algal Compounds or Extracts
2.1. Antibacterial Action
An array of diverse chemical compounds from algae, including alkaloids, polyketides, peptides, polysaccharides, phlorotannins, diterpenes, sterols, quinones, lipids, and glycerols, have been found to exhibit antibacterial action [14,31]. However, in some instances, the specific compounds with antibacterial properties are not fully elucidated, and the activity is collectively attributed to algal extracts. Also, as most studies focus on the antibacterial properties of algal extracts on human pathogens, information on plant pathogens is scarce but is constantly being enriched. An excellent example of algal extracts with antibacterial action comes from the brown marine alga Sargassum wightii (Table 1).
Table 1. Summary of algal compounds and their antibacterial activities.
| Algal Species | Compound/Type of Extract | Target Organism | Disease/Pathogenic Phenotype/Significance | Protected Plant/Organism | Mode of Action | Reference |
|---|---|---|---|---|---|---|
| Sargassum wightii | Methanolic extracts | Pseudomonas syringae | Leaf spot disease | Gymnema sylvestre | NK | [32] |
| Gracilaria edulis, Sargassum wightii, Enteromorpha flexuosa | Petroleum ether extracts, methanolic extracts, unsaponified and lipophilic fractions | Xanthomonas oryzae | Bacterial blight | Rice plants | NK | [16] |
| Sargassum wightii | Sulphoglycerolipid (methanol extract) | Xanthomonas oryzae | Bacterial blight | Rice plants | NK | [33] |
| Ulva fasciata | Methanolic extracts | Xanthomonas campestris, Erwinia carotovora | Plant pathogens | Various plant species | NK | [34] |
| Cystoseira myriophylloides, Fucus spiralis | Aqueous extracts | Agrobacterium tumefaciens | Crown gall disease | Solanum lycopersicum | NK | [18] |
| Sargassum latifolium, Hydroclathrus clathratus, Padina gymnospora | Methanolic extracts | Ralstonia solanacearum, Pectobacterium carotovorum | Brown rot disease | Potato plants | Induction of plant defenses, formation of bioactive secondary metabolites | [35] |
| Lessonia trabeculate, Macrocystis integrifolia | Ethanolic extracts | Erwinia carotovora Pseudomonas syringae | Plant pathogens | Various plant species (tomato, Arabidopsis, potato plants) | NK | [17] |
| Ulva lactuca, Gelidium serrulatum | Alkaline extracts | Xanthomonas vesicatoria | Plant pathogen | Tomato plants (in vitro) | Induction of plant defenses | [49] |
| Ulva lactuca, Sargassum filipendula, Gelidium serrulatum | Alkaline extracts | Xanthomonas vesicatoria | Plant pathogen | Tomato plants (in vivo) | Induction of plant defenses | [49] |
| Anabaena variabilis, A. circinalis | Ethyl acetate extracts | Aeromonas sp. | Skin infections, ulcers, hemorrhagic and septicemic infections | Fish | NK | [48] |
NK: not known.
The methanolic extracts of this species showed maximum antibacterial activity against the plant pathogen Pseudomonas syringae, to prevent leaf spot disease of the medicinal herb Gymnema sylvestre [32]. Similar in vitro activity against the phytopathogenic bacterium Xanthomonas oryzae was observed by the macroalgae Gracilaria edulis, Sargassum wightii, and Enteromorpha flexuosa [16]. The compound that was responsible for this action in the brown alga Sargassum wightii was the sulphoglycerolipid 1-O-palmitoyl-3-O(6′-sulpho-a-quinovopyranosyl)-glycerol (Figure 1) from the methanolic extract [33]. The plant pathogens Xanthomonas campestris and Erwinia carotovora were also inhibited in vitro by the methanol-soluble and insoluble extracts of Ulva fasciata [34]. The spray application of aqueous extracts from the brown algae Cystoseira myriophylloides and Fucus spiralis significantly reduced Crown gall disease incidence that was caused by the bacterial pathogen Agrobacterium tumefaciens in greenhouse tomato plants (Solanum lycopersicum) [18]. The response was related to oxidative burst mechanisms since the treated plants exhibited significantly greater activity levels of the plant defense enzymes polyphenol oxidase and peroxidase. The methanolic extract of the marine brown algae Sargassum latifolium, Hydroclathrus clathratus, and Padina gymnospora showed antibacterial activity against the soil-borne phytopathogenic bacteria Ralstonia solanacearum and Pectobacterium carotovorum. The extract of Padina gymnospora, which showed the most potent effect, was dominated by palmitic and oleic acids (Figure 1) [35].
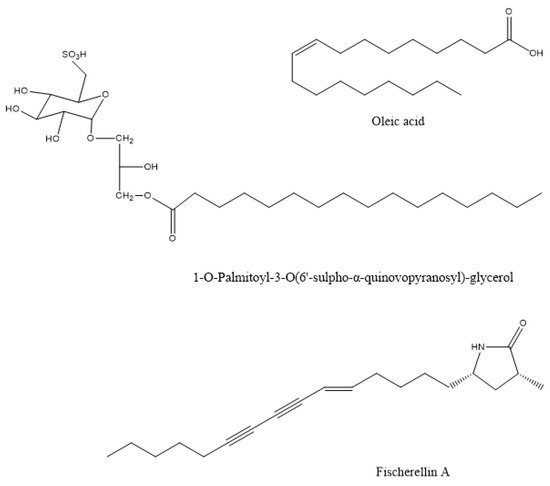
Figure 1. The chemical structure of active compounds that were isolated from Sargassum wightii, Padina gymnospora, and Fischerella muscicola.
In most documented cases, the antibacterial activity is against human or animal pathogens. In terms of agricultural practices, this is important for pathogenic bacteria that are associated with livestock. However, such agents could be further tested as potential candidates for plant protection applications, leaving ample room for future research [19,36,37,38,39,40,41,42,43,44,45,46,47]; such are the cases of the tetrabrominated diphenyl ether-like 2-(2′,4′-dibromophenoxy)-4,6-dibromoanisole (Figure 2) that was isolated from the green alga Cladaphora fascicularis that showed bactericidal action against Escherichia coli, Bacillus subtilis, and Staphylococcus aureus [38], and tetracyclic brominated diterpenes such as 3-hydroxy-1-deoxy-bromotetraspaerol (Figure 3) from the red alga Sphaerococcus coronopifolius that exhibited antibacterial activity against a panel of S. aureus strains [36]. Interestingly, ethyl acetate extracts from two cyanobacterial species, Anabaena variabilis and A. circinalis, were effective against bacterial fish pathogens belonging to the genus Aeromonas, proving to be useful in aquaculture applications [48]. Moreover, as most of the published studies focus on macroalgae or cyanobacteria’s antimicrobial activity, microalgae could constitute another exciting prospect for future research.
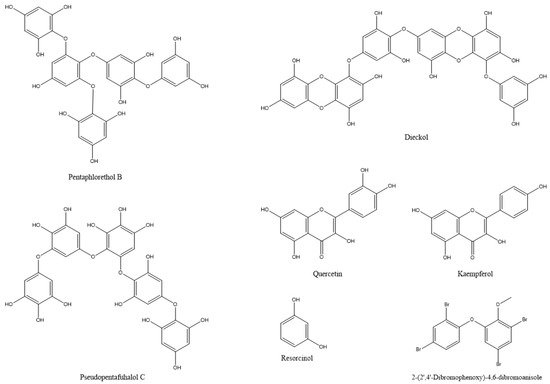
Figure 2. The chemical structures of active compounds that were isolated from Cladaphora fascicularis and Arthrospira platensis and other microalgae.
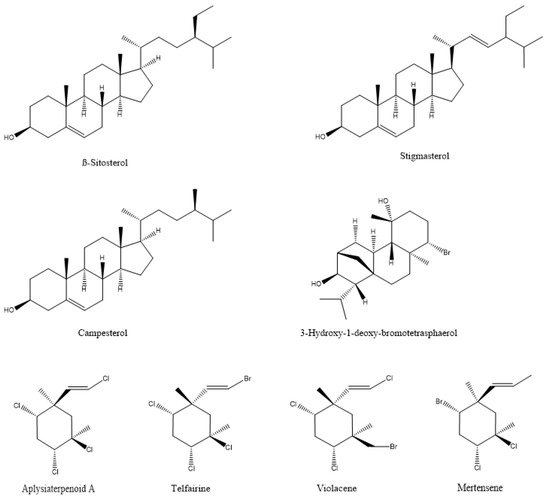
Figure 3. The chemical structure of active compounds that were isolated from Sphaerococcus coronopifolius, Plocamium cartilagineum, Plocamium telfairiae, and Prasiola crispa.
2.2. Antiviral Action
Plant viruses are a serious threat to agricultural crops, affecting product quality and yields and resulting in severe economic losses [30]. Their management is heavily dependent on synthetic chemical products, but natural compounds are continuously gaining ground. In this respect, natural compounds from algae that exhibit antiviral properties could be valuable resources. Among them are various polysaccharides, such as laminarins, agarans, alginate, carrageenans, and sulphated fucans, that can function as elicitors of defense mechanisms, as well as proteins, lipids, tannins, and terpenoids [10,13,30,50,51]. Polysaccharides are the most common compounds in algal extracts that induces antiviral responses in plants. For example, sodium alginate (Figure 4) from marine algae exhibited strong inhibitory activity against the tobacco mosaic virus (TMV) that was isolated from systemically-infected leaves of Nicotiana tabacum L. var bright yellow [21] (Table 2).
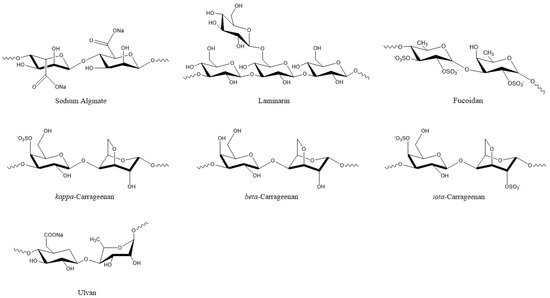
Figure 4. Algal polysaccharides with pesticidal action.
Table 2. Summary of the antiviral activities of algal compounds against plant pathogens.
| Algal Class/Species | Compound/Type of Extract | Target Organism | Disease/Pathogenic Phenotype | Protected Plant/Organism | Mode of Action | Reference |
|---|---|---|---|---|---|---|
| Phaeophyceae (brown seaweeds) | Sodium alginate | Tobacco mosaic virus (TMV) | Mottling and discoloration on leaves | Nicotiana tabacum | Aggregation of viral particles, blocking of decapsulation process | [21] |
| Tichocarpus crinitus | Kappa/beta-carrageenan | Tobacco mosaic virus (TMV) | Mottling and discoloration on leaves | Nicotiana tabacum | Plant tissue resistance, effect on the plant genome | [52] |
| Tichocarpus crinitus | Kappa/beta-carrageenan | Potato virus X (PVX) | Crinkle symptoms/plant death | Datura stramonium | Stimulation of lytic processes | [22] |
| Fucus gardneri, Alaria marginata, Ralfsia sp., Codium fragile, Fragilaria oceanica, Egregia menziesii | Methanolic extract (alginate) | Potato virus X (PVX) | Crinkle symptoms/plant death | Chenopodium quinoa | Aggregation of viral particles | [23] |
| Ulva pertusa | Lectins | Tobacco mosaic virus (TMV) | Mottling and discoloration on leaves | Nicotiana glutinosa, Chenopodium amaranticolor | NK | [53,54] |
| 13 species tested – Cystoseira balearica, Lophocladia lallemandii, and Gastroclonium clavatum exhibited the strongest effect |
Lipid extracts | Tobacco mosaic virus (TMV) | Mottling and discoloration on leaves | Nicotiana tabacum | NK | [19] |
| Durvillaea antarctica | Aqueous and ethanolic extracts | Tobacco mosaic virus (TMV) | Mottling and discoloration on leaves | Nicotiana tabacum | NK | [17] |
| Ulva clathrata, Cladosiphon okamuranus | Sulphated polysaccharides | Newcastle disease virus (NDV) | Respiratory infection, enteric disease, mortality | Poultry | Inhibition of cell–cell fusion | [55] |
NK: not known.
The degree of inhibition increased with alginate concentration and was higher when the alginate polymer had a lower mannuronate to guluronate ratio [21]. Another polysaccharide, i.e., kappa/beta-carrageenan (Figure 4) from red marine alga Tichocarpus crinitus, reduced tobacco mosaic virus (TMV) infection in Xanthi-nc tobacco leaves by 87 percent [52]. The same compound stimulated lytic processes against PVX particles in the leaves of Datura stramonium [22]. Methanolic extracts from six algal species also showed inhibitory effects against PVX by more than 80%. Among them, the extract from Fucus gardneri contained the polysaccharide alginate, which showed 95% success in suppressing PVX infection via aggregation of the virus particles [23].
Apart from polysaccharides, lectins (carbohydrate-binding proteins) that were isolated from the marine alga Ulva pertusa also showed antiviral action against tobacco mosaic virus (TMV) [53,54]. Similarly, lipids that were extracted from 11 algal species displayed antiviral activity against TMV on Nicotiana tabacum cv. Xanthi-nc. The brown alga Cystoseira balearica and the red alga Lophocladia lallemandii showed the highest inhibitory activity [19]. Aqueous and ethanolic extracts from the brown alga Durvillaea antarctica suppressed damage that was caused by TMV in tobacco leaves [17]. Moreover, an exciting application in livestock animals refers to the use of sulphated polysaccharides from the algae Ulva clathrata and Cladosiphon okamuranus in inhibiting Newcastle Disease Virus (NDV) infection in poultry [55].
Research for algal compounds with antiviral effects in plants has yielded significant results in the past years and remains promising for discovering new biopesticide products. However, as expected, algal antiviral research is mainly concentrated around human pathogens. Algal extracts have been studied extensively for their inhibitory action against important human viruses, such as human immunodeficiency virus (HIV), human papilloma virus (HPV), hepatitis B virus (HBV), herpes simplex virus types 1 and 2 (HSV-1, HSV-2), and diverse strains of Dengue virus (DENV-2) [56,57,58,59,60,61,62,63,64], suggesting that there are still many research opportunities in the field of algal antiviral products.
2.3. Antifungal Action
Natural algal compounds are constantly gaining ground in modern agricultural practices in controlling fungal infection, one of the most common types of disease in cultivated plants. They are mostly preferred over synthetic products, due to lower environmental impact, high specificity, and performance [14,51]. Algal powders and a large variety of extracts, such as aqueous, methanolic, ethanolic, diethyl ether, acetone, ethyl acetate, benzene, and chloroform, have proven to be effective in protecting plants against pathogenic fungal species [17,18,20,24,35,65,66,67,68,69,70,71,72,73,74,75,76,77]. For instance, ethanolic extracts of the cyanobacterium Nostoc strain ATCC 53,789 inhibited the growth of nine fungal plant pathogens (Table 3).
Table 3. Antifungal activity of algal extracts against known plant pathogens.
| Algal Species | Compound/Type of Extract | Target Organism | Disease/Pathogenic Phenotype/Significance | Protected Plant/Organism | Mode of Action | Reference |
|---|---|---|---|---|---|---|
| Nostoc sp. | Ethanolic extracts | Armillaria sp., Fusarium oxysporum f. sp. melonis, Penicillium expansum, Phytophthora cambivora, P. cinnamomi, Rhizoctonia solani, Rosellinia, sp., Sclerotinia sclerotiorum, Verticillium albo-atrum | Plant pathogens | In vitro (action against Sclerotinia sclerotiorum was verified in the presence of tomato plant). | Induction of plant defenses | [20] |
| Cystoseira myriophylloides, Laminaria digitata, Fucus spiralis | Aqueous extracts | Verticillium dahliae | Verticillium wilt disease | Tomato seedlings | Induction of plant defenses | [18] |
| Lessonia trabeculata | Ethanolic extracts | Botrytis cinerea | necrotic lesions in leaves | Tomato plants | NK | [17] |
| Gracillaria chilensis | Aqueous and ethanolic extracts | Phytophthora cinnamomi | Plant pathogen | In vitro | NK | [17] |
| Sargassum latifolium, Padina gymnospora | Methanolic extracts | Fusarium solani, Rhizoctonia solani | Plant pathogens | In vitro, in vivo (Solanum melongena) | Induction of plant defenses, formation of bioactive secondary metabolites | [35] |
| Ulva lactuca, Sargassum filipendula, Gelidium serrulatum | Alkaline extracts | Alternaria solani | Plant pathogen | Tomato plants | Induction of plant defenses | [49] |
| Laminaria digitata, Undaria pinnatifida, Porphyra umbilicalis, Eucheuma denticulatum Gelidium pusillum | Fatty acids, polysaccharides, phlorotannins | Botrytis cinerea, Monilinia laxa, Penicillium digitatum | Postharvest pathogens | In vitro, in vivo (Fragaria × ananassa, Prunus persica, Citrus limon) | Direct toxicity of fatty acids, induction of plant defenses | [68] |
| 10 algal species - Cystoseira balearica, Codium effusum and Codium coralloides exhibited the strongest effect |
Lipid extracts | Phoma tracheiphila | Mal secco disease | In vitro | NK | [19] |
| Ulva fasciata | Ulvan | Fusarium oxysporum f. sp. phaseoli | Bean Fusarium wilt | Phaseolus vulgaris | Induction of plant defenses, reduced fungal colonization in plant tissues | [78] |
| Ulva fasciata | Ulvan | Colletotrichum lindemuthianum | Anthracnose | Phaseolus vulgaris | Induction of plant defenses | [79] |
| Ulva armoricana | Aqueous extracts (ulvan) | Erysiphe polygoni, E. necator, Sphareotheca fuliginea | Plant pathogens | Phaseolus vulgaris, grapevine plants, Cucumis sativus | Induction of plant defenses | [80] |
| Ulva fasciata | Ulvan | Blumeria graminis | Plant pathogen | Triticum aestivum cv. Kanzler, Hordeum vulgare cv. Villa | Induction of plant defenses | [82] |
| Ulva fasciata | Sulphated polysaccharides, alcoholic extracts | Colletotrichum lindemuthianum | Anthracnose | In vitro, Phaseolus vulgaris | Induction of plant defenses | [83] |
| Laminaria digitata | Laminarin | Botrytis cinerea, Plasmopara viticola | Plant pathogens | Grapevine plants | Induction of plant defenses | [85] |
| Anabaena sp., Ecklonia sp., Jania sp. | Aqueous extracts containing polysaccharides | Botrytis cinerea | Grey mold (postharvest plant pathogen) | Strawberry plants | Direct effect, induction of plant defenses | [86] |
| Ulva lactuca, Caulerpa sertularioides, Padina gymnospora, Sargassum liebmannii | Polysaccharide-rich extracts | Alternaria solani | Plant pathogen | Tomato plants | Induction of plant defenses (Ulva lactuca) | [87] |
| Ulva sp. | Ulvan | Colletotrichumgloeosporioides | Glomerella leaf spot (GLS) disease | Apple plant seedlings (Malus domestica) | Induction of plant defenses | [88] |
| Ulva fasciata Enteromorpha flexuosa | Ethyl acetate, benzene, acetone, methanolic and chloroformic extracts | Macrophomina phaseolina Fusarium solani | Plant pathogens | Cucumber plants | NK | [89] |
| Gracilaria confervoides | Chloroformic extracts | Rhizoctonia solani, Fusarium solani, Macrophomina phaseolina | Plant pathogens | Cucumber plants | NK | [75] |
| Sargassum vulgare | Methanolic extracts | Pythium aphanidermatum | Pythium leak disease | Potato plants | NK | [90] |
| Sargassum wightii | Acetone extracts (n-Hexadecanoic acid) | Rhizoctonia solani | Rice sheath blight | Rice plant | Induction of plant defenses | [91] |
NK: not known.
The extract, which was directly applied on tomato plants, completely inhibited the growth of the fungus Sclerotinia sclerotiorum [20]. The aqueous extracts of three brown algae Cystoseira myriophylloides, Laminaria digitata, and Fucus spiralis, that were applied as spray or drench, had a protective effect against Verticillium wilt disease that was caused by the fungus Verticillium dahliae, in greenhouse tomato seedlings [18]. In tomato leaves that were infected with Botrytis cinerea, organic extracts from the brown alga Lessonia innamomic reduced the frequency and extent of necrotic lesions, while aqueous and ethanolic extracts from the red alga Gracillaria chilensis were effective against Phytophthora innamomic, showing dose and time-dependent responses [17]. Methanolic extracts of the sea brown algae Sargassum latifolium and Padina gymnospora showed antifungal activity against phytopathogenic fungus species such as Fusarium solani and Rhizoctonia solani in addition to antibacterial activity [35]. Notably, the antifungal activity of alkaline extracts of Ulva lactuca, Sargassum filipendula, and Gelidium serrulatum has been linked to the increase in plant defense enzymes activity and the overexpression of key marker genes of plant defense pathways [49].
De Corato et al. [68] performed in vitro and in vivo tests of extracts from two brown and three red macroalgae against three phytopathogenic fungi. The compounds that were identified in the extracts included twenty fatty acids, three types of polysaccharides (laminarans, fucoidans, and alginates) (Figure 4), and three types of phlorotannins (phlorethols, fucophloretols, and eckols) (Figure 2). Regarding lipids, palmitic acid, linoleic acid, and arachidonic acid were found at the highest concentrations in most extracts. The quantity of fatty acids corresponded to most of the total dry weight of the crude extracts and could be linked to the antifungal activity [68]. Lipids that were extracted from 10 algal species inhibited the germination of Phoma tracheiphila, a pathogenic fungus that causes a disease known as Mal secco on citrus trees. More specifically, the inhibition effect of the brown alga Cystoseira balearica and the green alga Codium effusum reached 100% [19].
Various algal oligo- or polysaccharides have demonstrated antifungal activity against plant pathogens either directly or indirectly, by activating plant defense mechanisms [78,79,80,81,82,83,84]. The cell wall polysaccharide laminarin (Figure 4) that was purified from the brown algae Laminaria digitata induced a defense response in grapevine leaves against the fungus Botrytis cinerea, effectively reducing the infection. In addition, grapevine plants that were sprayed on the leaves with laminarin were protected against the fungus Plasmopara viticola [85]. Polysaccharides from Anabaena sp., Ecklonia sp., and Jania sp. showed inhibitory action against Botrytis cinerea, effectively protecting strawberry fruits from infection in in vitro experiments [86]. Similar polysaccharide-rich extracts from green (Ulva lactuca and Caulerpa sertularioides) and brown (Padina gymnospora and Sargassum liebmannii) macroalgae induced resistance in tomato plants against the necrotrophic fungus Alternaria solani [87]. The water-soluble heteropolysaccharide ulvan (Figure 4) from Ulva sp. extracts significantly reduced the severity of Glomerella leaf spot (GLS) disease, caused by the fungus Colletotrichum gloeosporioides on the leaves of apple plant seedlings (Malus domestica). The induced resistance was associated with increased peroxidase activity, revealing that although ulvan does not exhibit antimicrobial activity, its action is associated with plant defense mechanisms [88].
Extracts and powders of the green seaweeds Ulva fasciata and Enteromorpha flexuosa inhibited the growth or affected the microsclerotia formation of the soil-borne fungi, Macrophomina phaseolina and Fusarium solani, that infect cucumber plants. The identification of iron-monocarbonyl and their functional groups, such as amine and ether in the extracts, could suggest a potential antifungal role for these compounds [89]. Similar compounds were also identified in the chloroform extracts from the red macroalgae Gracilaria confervoides that exhibited inhibitory action against three soil-borne pathogenic fungi of cucumber: Rhizoctonia solani, Fusarium solani, and Macrophomina phaseolina [75].
The methanolic extract of the brown alga Sargassum vulgare contained phenolic acids and flavonoids that may be responsible for the antifungal action that was observed against Pythium aphanidermatum, the causative agent of Pythium leak disease in potato [90]. Phenolic acids and phytoalexins were among the 18 compounds that were detected in the acetone extract of the brown alga Sargassum wightii. The extract exhibited antifungal activity against Rhizoctonia solani, the causative agent of rice sheath blight [91].
2.4. Nematocidal Action
The extracts and compounds from micro- and macroalgae are also effective against plant-parasitic nematodes that are responsible for the annual loss of 10–25% of worldwide crop production [92] (Table 4).
Table 4. Summary of algal products and their pesticidal activity against soil nematodes.
| Algal Species | Compound/Type of Extract/Product | Target Organism | Protected Plant/Organism | Mode of Action | Reference |
|---|---|---|---|---|---|
| Jania rubens | Brominated diterpenes | Allolobophora caliginosa | In vitro | ΝΚ | [93] |
| Nostoc sp. | Methanolic extracts | Caenorhabditis elegans | In vitro | Induction of plant defenses | [20] |
| Spatoglossum variabile, Stokeyia indica, Melanothamnus afaqhusainii | Dry powders | Meloidogyne incognita | Eggplant, watermelon | Direct cytotoxic effect, effect on plant metabolism/resistance to stress | [65] |
| Spatoglossum variabile, Melanothamnus afaqhusainii, Halimeda tuna | Aqueous and ethanolic extracts | Meloidogyne javanica | Sunflower, tomato | Induction of plant defenses | [24] |
| Sargassum tenerrimum, S. swartzii, S. wightii | Ethanolic extracts (dry powders) | Meloidogyne javanica | Okra (Abelmoschus esculentus) | ΝΚ | [94] |
| Stoechospermum polypodioides | Methanolic extracts | Meloidogyne javanica | In vitro | ΝΚ | [95] |
| Ecklonia maxima | Commercial formulation—Kelpak 66 liquid concentrate (cancelled product) | Meloidogyne incognita | Tomato plants (Lycopersicon esculentum) | ΝΚ | [96] |
| Ascophyllum nodosum, Ecklonia maxima | Commercial formulations—Kelpak (Kelp Products Ltd., Simon’s Town, South Africa), OSMO® (OSMO® International NV, Diksmuide, Belgium) | Meloidogyne chitwoodi, Meloidogyne hapla | Tomato plants (Lycopersicon esculentum) | Interrupt enzymatic activities of hatching process, alter sensory perception of the roots by the nematodes | [97] |
| Ascophyllum nodosum | Commercial formulation—Algaefol® (Chema Industries, Egypt) | Radopholus similis, Meloidogyne incognita, Belonolaimus longicaudatus | Citrus, tomato, centipede grass | Cytotoxic effect | [98,99,100,101] |
NK: not known.
Brominated diterpenes that were isolated from the marine red alga Jania rubens were effective against Allolobophora caliginosa [93]. Different concentrations of methanolic extracts from the Nostoc strain ATCC 53,789 either killed or slowed the development of the nematode C. elegans [20]. Dry powders from three macroalgae species, Spatoglossum variabile, Stokeyia indica, and Melanothamnus afaqhusainii displayed a suppressive effect on the root-knot nematode Meloidogyne incognita by reducing the gall formation and preventing nematode penetration in the roots of eggplant and watermelon [65]. Similar effects against the nematode Meloidogyne javanica were identified in okra, sunflower, and tomato after treatment with aqueous and ethanolic extracts of the marine macroalgae Sargassum tenerrimum, S. swartzii, S. wightii, Spatoglossum variabile, Melanothamnus afaqhusainii, and Halimeda tuna [24,94]. Algal extracts showed relatively similar suppressive effects with a carbofuran nematicide; however, the best result was obtained when extracts from Spatoglossum variabile were applied together with the synthetic product [24]. The marine alga Stoechospermum polypodioides was also effective against Meloidogyne javanica, causing 80% mortality, the strongest effect among 21 species of algae that were examined for their nematocidal action [95]. A reduction in root-knot nematode infestation using macroalgal extracts has been previously reported in tomato [96]. There are two commercially available products that are derived from the marine macroalgae Ascophyllum nodosum and Ecklonia maxima, that affected the hatching and sensory perception of the root-knot nematodes Meloidogyne chitwoodi and M. hapla. The alkaline extract from the brown marine alga A. nodosum showed a stronger inhibitory effect compared to the extract from the brown alga E. maxima, which in certain cases enhanced the infectivity of the nematodes [97]. Extracts of A. nodosum have shown inhibitory action against other nematodes, such as Radopholus similis, Meloidogyne incognita, and Belonolaimus longicaudatus, infecting citrus, tomato, and centipede grass, respectively [98,99,100,101].
2.5. Insecticidal—Acaricidal Action of Algae
Marine macroalgae extracts exhibit insecticidal/acaricidal activity and can be used in integrated pest management applications as environmentally friendly approaches for arthropod population control. These botanical biopesticides are safer than synthetic products and are equally or more effective since they display different modes of action. Therefore, their targets are less likely to develop resistance against them [102,103]. There are a large number of publications describing the pesticidal or repellent action of algal extracts against different arthropods that are either agricultural pests or related to human and animal health [25,26,104,105,106,107,108,109,110,111,112,113,114,115]. The bioactive compounds that were identified in the extracts, as expected, cover a broad range of chemical structures, including polysaccharides, phenolics, proteins, terpenes, lipids, and halogenated compounds [116]. For example, two halogenated monoterpenes (mertensene and violacene) (Figure 3) that were extracted from the red alga Plocamium cartilagineum, and the mertensene derivatives, dibromomertensene, and dihydromertensene, showed strong insecticidal activity against the tomato moth Tuta absoluta and the cereal aphid, Schizaphis graminum [104] (Table 5).
Table 5. Summary of algal products and their activity against insects and mites.
| Insecticidal Activity | ||||||
|---|---|---|---|---|---|---|
| Algal Species | Compound/Type of Extract | Target Organism | Disease/Significance | Protected Plant/Organism | Mode of Action |
Reference |
| Caulerpa racemosa | Ethanol and water extracts | Anopheles stephensi, Aedes aegypti, Culex quinquefasciatus | Disease vectors | - | Toxic effect (larvicidal) | [25] |
| Plocamium cartilagineum | Mertensene, violacene, and derivatives (dibromomertensene and dihydromertensene) | Tuta absoluta, Schizaphis graminum | Crop pests | Tomato plants, cereals | Toxic effect (insecticidal, reduced reproduction) | [104] |
| Spirulina platensis, Sargassum vulgar | Water and ethanol extracts | Spodoptera littoralis | Crop pest | Cotton plants, tomato, maize etc. | Toxic effect | [27] |
| Caulerpa sertularioides, Laurencia johnstonii, Sargassum horridum | Ethanol extracts | Diaphorina citri | Citrus greening disease | Citrus plants | Toxicity, repellent activity | [109] |
| Ulva lactuca | acetone, ethanol, chloroform, methanol, petroleum ether extracts | Culex pipiens, Spodoptera littoralis | Disease vector, crop pest | - | Inhibition of adult emergence and larval growth |
[117] |
| Caulerpa scalpelliformis | Chloroform, methanol, hexane extracts | Dysdercus cingulatus, Spodoptera litura | Crop pests | Cotton seeds, tomato, maize, vegetables | Repellent activity | [118] |
| Padina pavonica | Chloroform, benzene extracts | Dysdercus cingulatus | Crop pest | Cotton, citrus, maize | Toxic effect (nymphicidal, ovicidal) | [115] |
| Sargassum tenerrimum | Chloroform, benzene extracts | Dysdercus cingulatus | Crop pest | Cotton, citrus, maize | Toxic effect (nymphicidal, oviposition efficacy) | [114] |
| Ulva fasciata, U. lactuca | Methanol extracts | Dysdercus cingulatus | Crop pest | Cotton, citrus, maize | Toxic effect (nymphicidal) | [105] |
| Nostoc sp. | Methanol extracts | Helicoverpa armigera | Crop pest | Cotton, tomato, rice etc. | Toxic effect (larvicidal) | [20] |
| Sargassum wightii, Padina pavonica | Chloroform, methanol, water extracts | Dysdercus cingulatus | Crop pest | Cotton, citrus, maize | Toxic effect (nymphicidal), effect on biophysical parameters | [106] |
| Dictyota linearis, Padina minor | Ethanol extracts | Aedes aegypti | Disease vector | - | Toxic effect (larvicidal) | [107] |
| Caulerpa scalpelliformis | Acetone extract | Culex pipiens | Disease vector | - | Toxic effect (larvicidal) | [108] |
| Microcystis, Oscillatoria, Nodularia, Nostoc, Anabaena | Hydrophilic, lipophilic extracts | Aedes aegypti | Disease vector | - | Toxic effect | [110] |
| Ulva lactuca | Acetone extract | Drosophila melanogaster | Fruit fly, model organism | - | Toxic effect | [111] |
| Chara vulgaris, Parachlorella kessleri, Ulva intestinalis, Cladophora glomerata, Nostoc carneum | Ethanol extracts | Spodoptera littoralis | Crop pest | Cotton, tomato, maize, vegetables | Toxic effect (larvicidal), effect on biophysical parameters | [113] |
| Acaricidal activity | ||||||
| Ascophyllum nodosum | Commercial formulation—Maxicrop® (Maxicrop International Ltd.) | Tetranychus urticae | Mottled leaves, early leaf loss | Strawberry plant | - | [26] |
| Oscillatoria sp., Phormidium sp., Spirulina platensis, Spirulina maxima, Ulva intestinalis, Sargassum sp., Dictyota sp. | Methanol, dichloromethane, hexane extracts | Dermatophagoides pteronyssinus | Disease vector | - | Toxic effect | [112] |
Water and ethanol extracts from the cyanobacterium Arthrospira platensis (syn. Spirulina platensis) and the brown alga Sargassum vulgar contained phenols, tannins, and alkaloids. Further analysis of phenols in A. platensis revealed the presence of the phenolic compounds: quercetin, kaempferol, and resorcinol (Figure 2), which could be related to the insecticidal action of the extracts [27]. An evaluation of the ethanolic extracts of the macroalgal species (Caulerpa sertularioides, Laurencia johnstonii, and Sargassum horridum) revealed insecticidal and repellent activities against Asian citrus psyllid adults (Diaphorina citri). The chemical composition of the three extracts showed phenols, alkaloids, terpenes, tannins, flavonoids, saponins, and anthraquinones, which are associated with insecticidal and repellent activity [109]. In certain cases, algal extracts exhibit different insecticidal effects, depending on the insect species and on the developmental stages of the target insect. For instance, the acetone extract of Ulva lactuca was the most potent extract against Culex pipiens. In the case of the cotton leafworm, Spodoptera littoralis, ethanolic and chloroform extracts acted as larvicides, methanolic and ethanolic extracts resulted in the highest pupation inhibition, whereas etheric and methanolic extracts strongly inhibited larval growth and adult emergence [117]. In terms of agricultural pests, more research has focused on the insecticidal activity of algal extracts against the cotton insect pests of the genera Dysdercus and Spodoptera. Algal extracts of various species, including Caulerpa scalpelliformis [118], Padina pavonica [115], Sargassum tenerrimum [114], Sargassum vulgar [27], Ulva fasciata, and U. lactuca [105] were effective against these agricultural pests by causing nymphal mortality, adult mortality, abnormal development, or reducing adult lifespan, fecundity, and hatchability [14,106]. As observed throughout the text, algal extracts could be used against more than one target type (antimicrobial, insect, mite, etc.). Therefore, apart from the antifungal action, the methanolic extracts of the cyanobacterium Nostoc strain ATCC 53,789 also killed larvae of the moth Helicoverpa armigera [20].
The volatile oils from Actinotrichia fragilis, Liagora ceranoides, and Colpomenia sinuosa were hydrodistilled and contained different aliphatic alcohols and long-chain hydrocarbons as the major components. They caused 55–90% and 60–80% mortality to pests of stored products Oryzaephilus mercator and Tribolium castaneum, respectively, at a dose of 12 μL/L air after 48 h of fumigation exposure [119].
The volatile oils of A. fragilis caused 80–90% mortality in both T. castaneum and O. mercator and consisted of 49% aliphatic alcohols, mainly 1-dodecanol (39.6%) [119]. The insecticidal activity of 1-dodecanol was suggested to be related to the developing cuticle, producing a disruption in the cuticular tanning process [120].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10020307
This entry is offline, you can click here to edit this entry!
