Colour is one of the most relevant organoleptic attributes that directly affects consumers’ acceptance and food selection. However, as food colouring pigments are generally unstable and become modified during processing, in order to maintain or restore product colour uniformity, colourants are added to food products around the world. Food properties, namely colours, which are a visual feature associated with the spectral distribution of light resulting from the interaction with matter, largely determine consumer’s satisfaction and expectations, affecting their choice and eating desires. Food colours affect recognition and product acceptability (warning consumers against eating spoiled food which is hazardous to health), as they are a beam of sensory perceptions such as sight, smell, and taste.
1. Natural Food Colours and Food Colours Synthesized Equally to the Natural
Food, as a basic need for human consumers, must be wholesome and safe. However, in this context, colour is also one of the most impressive attributes of foodstuffs, which directly determines the preference, selection and eating desires of the consumers. Still, the utilization of colouring additives in food is confronted with debate. On a global scale, the use of colours in food has faced challenges with disagreement, particularly when added at high doses (i.e., when exceeding the recommended doses).
Curcumin (E100), also named as “Cl natural yellow 3”, “yellow–saffron”, “turmeric yellow” or “diferoyl methane” [
7] is obtained from the rhizome of
Curcuma longa L., and furnishes a yellow or orange-yellow colour to food products. This food colour consists essentially of curcumin (
Figure 1), two other derivatives at different proportions, and small amounts of naturally occurring oils and resins in the raw material. Curcumin is insoluble in water and diethyl ether, but soluble in ethanol and glacial acetic acid [
22].
Figure 1. Structural formula of curcumin (E100).
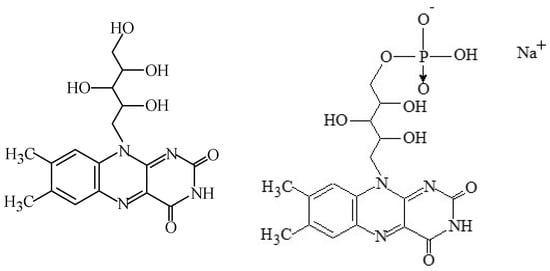
Riboflavin, also identified as “lactoflavin” or “vitamin B
2” (C
17H
20N
4O
6), and “riboflavin-5’-phosphate” (C
17H
20N
4NaO
9P·2H
2O) are water-soluble colourants synthesized by plants and several microorganisms. They are essential micronutrients in the human diet, acting as precursors to flavine adenine dinucleotide and flavin mononucleotide, which function as hydrogen carriers in biological redox processes. These food colours are yellow or orange-yellow crystalline powders, with a slight odour [
7,
36], with the codes E101(i) and E101(ii), respectively (
Figure 2). Although it has been known for many years that bacteria using fermentation technology could produce riboflavin, only recently has a pure product been obtained using a genetically modified strain of
Bacillus subtilis or the fungus
Ashbya gossypii.
Figure 2. Structural formulae of riboflavin (E101i) and riboflavin-5’-phosphate (E101ii).
Cochineal, carminic acid or carmines, also named “Cl natural red 4”, (with the chemical formula C
22H
20O
13) is a food colour identified as E120, which is freely soluble in water [
38] (FAO, 2000) and shows a red colour [
7,
39].
Carmines and carminic acid (
Figure 3) can be obtained from aqueous, aqueous alcoholic or alcoholic extracts of cochineal, and consist of the dried bodies of the female insect
Dactylopius coccus Costa. The insects of the
Coccidae family are parasites of some species of cacti. During the last century, the Canary Islands were the main production centre, but today this product can be obtained in large quantities in Peru and other countries of America. The insects are so small that about 100,000 are need to obtain 1 kg of product. However, they are very rich in the food colourant, reaching up to about 20% of their dry weight. The chemical principle of this colourant is the carminic acid, but the substance obtained by extraction with hot water (from insects) alone has no colour. The food colourant itself is obtained through aluminum or calcium’s addition to this extracted product. For some applications, especially beverages, ammonia is added instead of metal [
1,
2]. Aluminum lakes of carminic acid (carmines) can be formed (i.e., these substances are thought to be present in the molar ratio 1:2). In commercial products, the colouring principle is associated with ammonium, calcium, potassium or sodium cations (singly or combined, with these cations eventually being in excess). Commercial products may also contain proteinaceous material derived from the insect source, and free carminate, or a small residue of unbound aluminum cations [
7].
Figure 3. Structural formula of carminic acid (E120).
The chloroplasts of higher plants have two types of chlorophyll (a and b), which are insoluble in water, soluble in alcohol, and sensitive to light, pH, oxygen and heat (Figure 4). Chlorophyll a prevails, but degrades more easily.
Figure 4. Structural formula of chlorophylls a and b.
Chlorophyll, with the code E140(i), can be extracted with acetone, methyl ethyl ketone, dichloromethane, carbon dioxide, methanol, ethanol, propan-2-ol and hexane [
7]. Heating foods containing chlorophyll (i.e., scalding vegetables before freezing or before they are canned) might lead to a colour change (to a more “pale” green), due to the loss of the magnesium atom in the chlorophyll molecule (which therefore determines pheophytins synthesis). Thus, the instability of chlorophylls determines their limited use as food additives, despite the absence of toxicity for the amounts usually consumed [
1,
2].
Caramels, with the code E150, are one of the oldest and most-often used colourants in foods and beverages. Caramels are often used in the food industry to impart or intensify the yellow or brown colour, being miscible with water (in liquid or powdered forms), but can also disperse in an oil system (producing pastes or emulsions). Four caramel food colours can be used—E150a, E150b, E150c, and E150d—and are prepared by caramelization (i.e., the controlled heat treatment of carbohydrates with food-grade reactants) but differ in some of the raw materials used in their preparation. During caramelization, carbohydrates incompletely decompose, dehydrate and polymerize at high temperatures (which is closely related to the temperature and type of carbohydrates), producing a mixture of chemical substances without specific compositions. For instance, at 160 °C, sucrose forms glucose and fructan, whereas at 185–190 °C isotopecane ((C
12H
24O
10)n) is synthetized, and, at about 200 °C, polymerization develops caramel alkane ((C
24H
36O
18)n) and caramel olefins ((C
36H
50O
25)n). Additionally, caramel alkyne ((C
24H
36O
13)n) occurs at 200 °C or more. All of these food colour caramels are described as dark brown to black liquids or solids, with an odour of burnt sugar [
7].
Vegetable carbon, also known as “vegetable black” (E153), is insoluble in water and organic solvents [
7], and is produced from green bamboo refined from through a high-temperature carbonization process with steam activation. The steam activation is achieved by charring the vegetable fibers of vegetable materials, such as wood, cellulose residues, peat, coconut husks and other fruits. The obtained residues are ground into small particles, with glycerin or glucose added for use in food products. Therefore, this additive, which takes the form of an odourless black powder, essentially consists of finely divided carbon, but can also contain small amounts of nitrogen, oxygen and hydrogen, and can adsorb other substances after preparation.
Carotenes are hydrocarbons with a skeleton of 40 carbon atoms; like xanthophylls, they belong to the family of carotenoids. Most of the physical and chemical characteristics of β-carotene (
Figure 5) are similar to the other family members of carotenoids [
1].
Figure 5. Structural formula of β-carotene (β,β-carotene), the main component of E160a.
Annatto bixin E160(i) and annatto norbixin E160(ii) can be obtained by extraction from the spiney seed pods of
Bixa orellana L. (also known as “Annatto” or ‘Achiote’ in large parts of South America, and as ‘Urucum’ in Brazil), and provide a yellow-to-red colour to food products [
10].
Bixin, which is a carotenoid, is extracted from the seed using hot vegetable oil. However, bixin is only soluble in vegetable oil at low percentage rates. Thus, stronger products can be obtained using bixin suspensions, by carrying out repeated extractions of annatto seeds (yielding bixin concentrations of ca. 4% or greater). Oil-soluble bixin is a yellow color, but its suspensions are a deep, vivid red-orange. By saponification, the methyl ester of bixin is cleaved, forming norbixin products, which are water-soluble colourants. Like bixin, norbixin varies in hue from yellow to orange, depending on the usage rate and application.
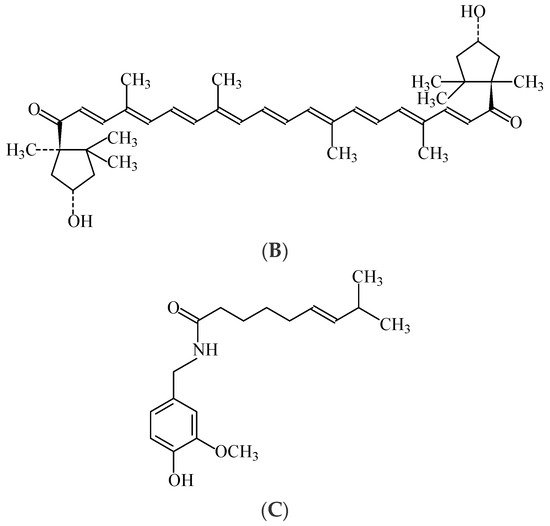
Paprika extract, also known as “capsanthin” or “capsorubin”, is a natural food colouring (E160c) in the form of a viscous, dark-red liquid. Capsanthin and capsorubin (
Figure 7) are the colourings that impart the yellow-to-orange hue characteristics of paprika. Paprika is extracted with acetone, ethanol, methanol, hexane, dichloromethane, ethyl acetate or carbon dioxide from ground fruits, with or without seeds, from the natural strains of
Capsicum annuum L. that contain capsanthin (C
40H
56O
3), capsorubin (C
40H
56O
4), and other lesser quantities of coloured compounds, namely xanthophyll, β-carotene and capsaicin [
7]. Paprika extract also contains a large amount of capsaicin (C
18H
27NO
3), which is a flavour component. This red spice imparts flavour, and the paprika color compounds can be solvent extracted to synthesize paprika oleoresin, a purified form of the colouring compounds. Paprika and paprika oleoresin are stable to heat but sensitive to light and alkaline conditions, and are insoluble in water.
Figure 7. Structure formulae of capsantin (A), capsorubin (B) (colored components of pepper extract), and capsaicin (C), the main flavouring component of chili extract.
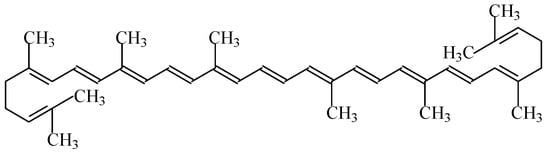
Lycopene, a bright red carotenoid, is a symmetrical tetraterpene (i.e., assembled from eight isoprene units). In its natural, all-
trans form, the molecule is long and straight, constrained by its system of 11 conjugated double bonds (
Figure 8), which reduces the energy required for electrons to transition to higher energy states, making possible the absorption of visible light with progressively longer wavelengths. Thus, as it absorbs the longest wavelengths of visible light, it displays a red colour. Lycopene (C
40H
56), exists as synthetic lycopene (E160d i), as lycopene from red tomatoes (E160d ii), and as lycopene from
Blakeslea trispora (E160d iii, also called “natural yellow 27”). The food colours E160d-i and E160d-iii are red crystalline powders, whereas E160d-ii is a viscous liquid with a dark red colour. Lycopene is insoluble in water, but freely soluble in chloroform [
7].
Figure 8. Structural formula of lycopene (E160d).
The carotenoid β-apo-8′-carotenal (C 30), also called “Cl 6 food orange” (E160e), is a natural, but chemically modified, food colouring (C30H40O), ranging from orange to dark red (Figure 9). This carotene aldehyde is resistant to temperature, does not decompose from light, preserves the shelf life of products, and restores products’ colour after heat treatment.
Figure 9. Structural formula of β-apo-8′-carotenal (E160e).
Lutein is a xanthophyll (C
40H
56O
2) of natural origin used as food colouring (E161b), which appears in the form of a dark, yellowish-brown liquid, and gives a reddish-yellow colour to food products. This food colour (
Figure 10), which is fat-soluble and a powerful anti-oxidant [
57], is obtained by extraction with acetone, methyl ethyl ketone, ethanol, methanol, 2-propanol, hexane, dichloromethane or carbon dioxide [
7] from natural varieties of edible fruits and plants, grass, lucerne (alfalfa) and
Targets erecta (i.e., although lutein also exists in eggs, it is not extracted from this product). Nevertheless, after removal, lutein extracts can also contain fats, oils and waxes from the original plants.
Figure 10. Structural formula of lutein (E161b).
Canthaxanthin (C
40H
52O
2) is a keto-carotenoid pigment which is widely distributed in nature (namely, in flamingo feathers, koi carp skin and crustacean shells) that was firstly isolated in edible mushrooms. This food colour (E161g), also named “Cl food orange 8” and known as β-Carotene-4,4′-dione and 4,4′-dioxo-β-carotene [
7], is a potent lipid-soluble antioxidant [
65,
66]. Although it is a natural product, for food colour purposes, canthaxanthin is usually synthesized.
Beetroot-red, also named “betanin” or “beet-red”, is a natural food colour (E162) displaying red to dark-red colours. It is a red glycosidic food dye obtained from the roots of red beets (
Beta vulgaris L. var. rubra), by pressing crushed beets, or by the aqueous extraction of shredded beet roots (the concentration of which can reach about 300–600 mg/kg) and subsequent enrichment in the active principle [
7].
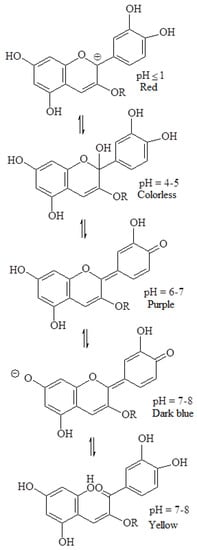
Anthocyanins are a group of natural water-soluble food colours (E163) that take on colours ranging from purple to blue, but can display other colours depending on the pH (
Figure 14) [
78]. Anthocyanins belong to a parent class of flavonoids synthesized via the phenylpropanoid pathway; although they occur in all tissues of higher plants, they can be obtained from edible vegetables and fruits, such as blueberries, blackberries, strawberries, currants, raspberries, and grapes.
Figure 14. Example of anthocyanin changes due to pH variation [
78].
Calcium carbonate (CaCO
3), also referred as “CI pigment white 18” or “chalk”, is a stable food colouring (E170) that does not require any specific processing to preserve its colouring properties. Additionally, calcium carbonate is also used as an acidity regulator, an anticaking agent (i.e., prevents food particles from sticking together) and a stabilizer (thus maintaining the uniform dispersal of substances in a food). This inorganic salt is a white crystalline or amorphous, odourless and tasteless powder, which is practically insoluble in water and ethanol [
7]. Calcium carbonate is a natural mineral derived from earth’s limestone, marble, or the sedimentation of crushed marine shells. For the food industry, the additive E170 is obtained by processing and cleaning chalk deposits.
Titanium dioxide (TiO
2), also named “CI pigment white 6”, is a metal oxide extracted from ilmenite, rutile and anatase. Titanium dioxide is use as a white food colouring (E171) due to its brightness and very high refractive index. Like all food colourants, its technological function is to make food more visually appealing, to give colour to food that would otherwise be colourless, or to restore the original appearance of a foodstuff. Titanium dioxide is also a nontoxic antimicrobial (i.e., it has potential bactericidal and fungicidal applications). E171, which is presented in the form of an odourless and tasteless white amorphous powder with pH 7, consists of pure titanium oxide; however, it can be coated with small amounts of alumina or silica to improve its technological properties. E171 is insoluble in water and organic solvents [
7].
Iron oxides and hydroxides (E172) can naturally be found in rusts, or can be artificially produced from iron sulphate.
Aluminum, also referred as “CI pigment meta”, can be used as a food colouring (E173l), being a silvery-grey powder or tiny sheets which are insoluble in water and in organic solvents [
7]. Aluminum powder consists of finely divided particles that eventually can be carried out in the presence of edible vegetable oils or fatty acids, and are used as food colours. This dye, which has a very limited use, can be applied in the external coating of sugar-based confectionery products for the decoration of cakes and pastries [
3].
Silver food colour (E174) is present in its elemental form, and is chemically obtained from the electrolysis of silver ore. It is a soft, white and lustrous transition metal, which exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. This coloured powder, or straw, is also a bactericide and a water-insoluble substance, and is only of use in coatings and decorations for cakes and sweets, liqueurs, and drinking water disinfection [
3]. Its acceptable daily intake has not been specified, but at a physiological level the elimination of E174 through the kidneys is slow, which can induce side effects of deposits at the tissue level [
16]. For instance, a high intake (a few grams) leads to poisoning. However, there is a lack of data regarding the toxicity and carcinogenic potential of the use of silver as a food additive [
93].
Gold can be applied as a food colour (E175), giving rise to a very unreactive metallic surface colour. It occurs in the form of golden-coloured powders, or straws, and is used in liqueurs and in the outer coating of confectionery and chocolate decorations [
3]. The acceptable daily intake for E175 has not been specified, as there are very limited data on the absorption, distribution, metabolism and excretion of elemental gold (as well as the toxicological aspects linked to its use as a food colour). Nevertheless, as elemental gold has a very low solubility, the systemic availability and effects might be low. At a physiological level, some side effects on health—namely disorders of the blood formula—are described [
16]. Nevertheless, although there are some studies with rats, the available data did not allow an evaluation of the genotoxic hazard associated to the use of gold as a food colour [
94].
2.2. Synthetic Food Colours
Synthetic food colours have been increasingly used rather than natural food colours by food manufacturers, as they have several economically relevant traits, such as their low cost; resistance to light, oxygen, and pH changes; and high colour stability. In contrast to natural food colours, which are usually extracted from several natural sources and purified, synthetic food colours are produce by full chemical synthesis or the modification of several precursor compounds. Besides this, they can be used without further transformation, and do not degrade during food processing.
This entry is adapted from the peer-reviewed paper 10.3390/foods11030379