Bast fiber crops are an important group of economic crops for the purpose of harvesting fibers from stems. These fibers are sclerenchyma fibers associated with the phloem of plants. They arise either with primary tissues from the apical meristem, or with secondary tissues produced by the lateral meristem. Fungal diseases have become an important factor limiting their yield and quality, causing devastating consequences for the production of bast fiber crops in many parts of the world.
- bast fiber crops
- molecular identification
- fungal disease
- DNA barcode
- PCR assay
1. Introduction
2. Bast Fiber Crops
|
Crop |
Main Distribution |
Main Characters of Growth Habitat |
Main Applications |
Main Fungal Diseases |
|---|---|---|---|---|
|
Flax (Linum usitatissimum Linnaeus) |
France, Russia, Netherlands, Belarus, Belgium, Canada, Kazakhstan, China, India |
Well-drained loam and cool, moist, temperate climates |
Linen, flax yarn, flax seed, linseed oil |
flax wilt, flax blight, flax anthracnose |
|
Hemp (Cannabis sativa Linnaeus) |
China, Canada, USA, Europe, East Asia, Nepal |
Grows at 16–27 °C, sufficient rain at the first six weeks of growth, short day length. |
Textiles, hempseed oil, prescription drugs |
hemp powdery mildew, hemp leaf spot disease, hemp blight, hemp root and crown rot wilt, hemp charcoal rot |
|
Jute (Corchorus capsularis Linnaeus) |
India, Bangladesh, Burma, China |
Tropical lowland areas, humidity of 60% to 90%, rain-fed crop |
Textiles, medicine |
jute anthracnose, jute brown wilt, jute leaf spot |
|
Kenaf (Hibiscus cannabinus Linnaeus) |
India, Bangladesh, China, Malaysia, Thailand |
Sandy loam and warm, humid subtropical, or tropical climates, few heavy rains or strong winds, at least 12 h light each day |
Textiles |
kenaf anthracnose, kenaf lack rot, kenaf sooty mold |
|
Ramie (Boehmeria nivea Linnaeus) Gaudich |
China, Brazil, Philippines, India, Vietnam, Laos, Cambodia |
Sandy soil and warm, wet climates, rainfall averaging at least 75 to 130 mm per month |
Textiles, soil and water conservation, medicine |
ramie anthracnose, ramie powdery mildew, ramie black leaf spot, ramie blight |
|
Sunn Hemp (Crotalaria juncea Linnaeus) |
India, USA, China |
Wide variety of soil condition, altitude from 100 to 1000 m, temperatures above 28 °C, photoperiod-sensitive |
Cover crop or green manure, forage producer |
sunn hemp fusarium wilt, sunn hemp root rot, sunn hemp powdery mildew |
3. Fungal Pathogens of Bast Fiber Crops
|
Pathogen |
Disease |
Method |
Marker |
Host Plant |
Geographic Region(s) |
Reference |
|
|---|---|---|---|---|---|---|---|
|
Alternaria |
|||||||
|
A. alternata |
Hemp leaf spot |
Conventional PCR |
ITS |
Cannabis sativa |
Shanxi, China |
[23] |
|
|
A. alternata |
Ramie black leaf spot |
Conventional PCR |
ITS, GAPDH |
Boehmeria nivea |
Hunan, Hubei, China |
[24] |
|
|
Cercospora |
|||||||
|
Cercospora cf. flagellaris |
Hemp leaf spot disease |
Not mentioned |
ITS, EF-1α, CAL, H3, actin |
Cannabis sativa |
Kentucky, USA |
[25] |
|
|
Colletotrichum |
|||||||
|
C. corchorum capsularis |
Jute anthracnose |
Conventional PCR |
ACT, TUB2, CAL, GAPDH, GS, and ITS |
Corchorus capsularis L. |
Zhejiang, Fujian, Guangxi, and Henan, China |
[26] |
|
|
C. fructicola |
Jute anthracnose |
Conventional PCR |
ACT, TUB2, CAL, GAPDH, GS, and ITS |
Corchorus capsularis L. |
Zhejiang, Fujian, Guangxi, and Henan, China |
[27] |
|
|
C. fructicola |
Jute anthracnose |
Conventional PCR |
ACT, TUB2, CAL, GAPDH, GS, and ITS |
Corchorus capsularis L. |
Zhejiang, Fujian, Guangxi, and Henan, China |
[26] |
|
|
C. gloeosporioides |
Ramie anthracnose |
Conventional PCR |
ITS |
Boehmeria nivea |
HuBei, HuNan, JiangXi, and SiChuan, China |
[28] |
|
|
C. higginsianum |
Ramie anthracnose |
Conventional PCR |
ITS |
Boehmeria nivea |
HuBei, China |
[29] |
|
|
C. phormii |
New Zealand flax anthracnose |
Conventional PCR |
ITS |
Phormium tenax |
California, USA |
[30] |
|
|
C. phormii |
New Zealand flax anthracnose |
Conventional PCR |
ITS |
Phormium tenax |
Perth, Australia |
[31] |
|
|
C. siamense |
Jute anthracnose |
Conventional PCR |
ACT, TUB2, CAL, GAPDH, GS, and ITS |
Corchorus capsularis L. |
Zhejiang, Fujian, Guangxi, and Henan, China |
[27] |
|
|
Colletotrichum sp. |
Kenaf anthracnose |
Conventional PCR |
ITS |
Corchorus olitorius |
South Korea |
[32] |
|
|
Curvularia |
|||||||
|
C. cymbopogonis |
Hemp leaf spot |
Conventional PCR |
25S |
Cannabis sativa |
USA |
[33] |
|
|
Exserohilum |
|||||||
|
E. rostratum |
Hemp floral blight |
Not mentioned |
ITS, RPB2 |
Cannabis sativa |
North Carolina, USA |
[34] |
|
|
Fusarium |
|||||||
|
F. oxysporum |
Hemp roots and crown rot |
Conventional PCR |
ITS, EF-1α |
Cannabis sativa |
Canada |
[35] |
|
|
F. oxysporum |
Jute brown wilt |
Conventional PCR |
ITS |
Corchorus olitorius |
Dhaka, Manikgonj, Kishorgonj, Rangpur, and Monirampur, Bangladesh |
[36] |
|
|
F. oxysporum |
Hemp wilt |
Conventional PCR |
ITS, EF-1α |
Cannabis sativa |
California, USA |
[37] |
|
|
F. solani |
Hemp crown root |
Conventional PCR |
ITS, EF-1α |
Cannabis sativa |
Canada |
[35] |
|
|
F. solani |
Hemp wilt |
Conventional PCR |
ITS, EF-1α |
Cannabis sativa |
California, USA |
[37] |
|
|
F. solani |
Sunn hemp root rot and wilt |
Conventional PCR |
ITS, EF-1α |
Crotalaria juncea |
Ceará, Brazil |
[38] |
|
|
F. brachygibbosum |
Hemp wilt |
Conventional PCR |
ITS, EF-1α |
Cannabis sativa |
California, USA |
[37] |
|
|
F. udum f. sp. crotalariae |
Sunn hemp fusarium wilt |
Conventional PCR |
EF-1α, β-tubulin |
Crotalaria juncea |
Tainan, China |
[39] |
|
|
Glomus |
|||||||
|
G. mosseae |
Hemp root rot |
Conventional PCR |
25S |
Cannabis sativa |
USA |
[33] |
|
|
Golovinomyces |
|||||||
|
G. spadiceus |
Hemp powdery mildew |
Not mentioned |
ITS, 28S |
Cannabis sativa |
Kentucky, USA |
[40] |
|
|
G. cichoracearum sensu lato |
Hemp powdery mildew |
Conventional PCR |
ITS |
Cannabis sativa |
Atlantic Canada and British Columbia. |
[41] |
|
|
G. cichoracearum |
Sunn hemp powdery mildew |
Not mentioned |
ITS |
Crotalaria juncea |
Florida, USA |
[42] |
|
|
Lasiodiplodia |
|||||||
|
L. theobromae |
Kenaf black rot |
Conventional PCR |
ITS |
Corchorus olitorius |
Kangar Perlis, Malaysia |
[43] |
|
|
Leptoxyphium |
|||||||
|
L. kurandae |
Kenaf sooty mould |
Conventional PCR |
ITS |
Corchorus olitorius |
Iksan, Korea |
[44] |
|
|
Macrophomina |
|||||||
|
Macrophomina phaseolina |
Hemp charcoal rot |
Conventional PCR |
EF-1α, CAL |
Cannabis sativa |
Southern Spain |
[45] |
|
|
Micropeltopsis |
|||||||
|
Micropeltopsis cannabis |
Unknown |
Conventional PCR |
25S |
Cannabis sativa |
USA |
[33] |
|
|
Orbilia |
|||||||
|
Orbilia luteola |
Unknown |
Conventional PCR |
25S |
Cannabis sativa |
USA |
[33] |
|
|
Pestalotiopsis |
|||||||
|
Pestalotiopsissp. |
Hemp spot blight |
Conventional PCR |
25S |
Cannabis sativa |
USA |
[33] |
|
|
Podosphaera |
|||||||
|
P. xanthii |
Ramie powdery mildew |
Conventional PCR |
ITS |
Boehmeria nivea |
Naju, Korea |
[46] |
|
|
Pythium |
|||||||
|
P. dissotocum |
Browning and a reduction in root mass, stunting |
Conventional PCR |
ITS, EF-1α |
Cannabis sativa |
Canada |
[35] |
|
|
P. myriotylum |
Browning and a reduction in root mass, stunting |
Conventional PCR |
ITS, EF-1α |
Cannabis sativa |
Canada |
[35] |
|
|
P. myriotylum |
Hemp root rot and Wilt |
Conventional PCR |
ITS, COI, COII |
Cannabis sativa |
Connecticut, USA |
[47] |
|
|
P. aphanidermatum |
Hemp root rot and crown wilt |
Conventional PCR |
ITS |
Cannabis sativa |
California, USA |
[37] |
|
|
P. aphanidermatum |
Hemp crown and root Rot |
Conventional PCR |
ITS |
Cannabis sativa |
Indiana, USA |
[48] |
|
|
P. ultimum |
Hemp crown and root Rot |
Conventional PCR |
ITS |
Cannabis sativa |
Indiana, USA |
[49] |
|
|
Rhizoctonia |
|||||||
|
Binucleate R. spp. |
Hemp wilt |
Conventional PCR |
25S |
Cannabis sativa |
USA |
[33] |
|
|
Sclerotinia |
|||||||
|
Sclerotinia minor |
Hemp crown rot |
Conventional PCR |
ITS |
Cannabis sativa |
San Benito County, Canada |
[50] |
|
|
Sphaerotheca |
|||||||
|
S. macularis |
Hemp powdery mildew |
Conventional PCR |
25S |
Cannabis sativa |
USA |
[33] |
|
|
Verticillium |
|||||||
|
V. dahliae |
flax wilt |
Conventional PCR |
ITS |
Linum usitatissimum |
La Haye Aubrée, France |
[51] |
|
|
V. dahliae |
flax wilt |
qPCR |
ITS |
Linum usitatissimum |
Normandy, France |
[52] |
|
|
V. dahliae |
flax wilt |
qPCR |
ß-tubulin |
Linum usitatissimum |
Germany |
[53] |
|
|
V. tricorpus |
flax wilt |
qPCR |
ITS |
Linum usitatissimum |
Germany |
[53] |
|
|
V. longisporum |
flax wilt |
qPCR |
ß-tubuIin |
Linum usitatissimum |
Germany |
[53] |
|
qPCR: quantitative PCR, ITS: internal transcribed spacer, GAPDH: glyceraldehydes-3-phosphate dehydrogenase, GS: glutamate synthetase, EF-1α: translation elongation factor 1-α, CAL: calmodulin, H3: histone subunit 3, ACT: actin, TUB2: ß-tubulin, RPB2: RNA polymerase subunit B2, COI: cytochrome oxidase subunit I, COII: cytochrome oxidase subunit II.
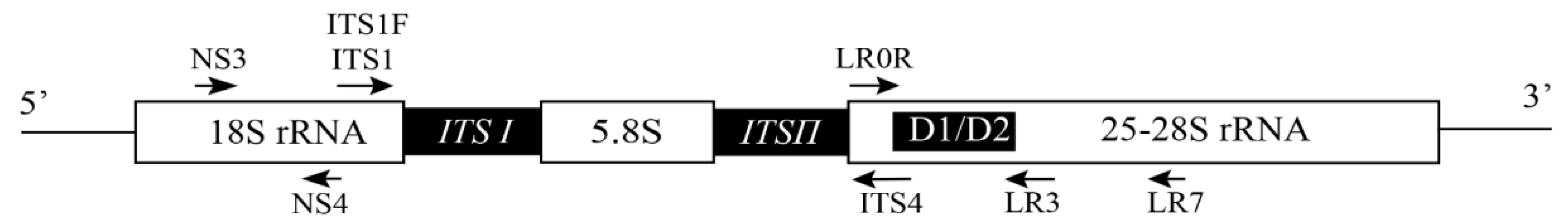
4. Development of Molecular Identification of Bast Fiber Fungal Pathogens
|
Target DNA |
Primer Name and Sequence (5′-3′) |
Size of PCR Product (bp) |
Reference |
|
|---|---|---|---|---|
|
18S |
NS3 |
GCAAGTCTGGTGCCAGCAGCC |
Not mentioned |
[54] |
|
NS4 |
CTTCCGTCAATTCCTTTAAG |
|||
|
28S |
LR0R |
GCAAGTCTGGTGCCAGCAGCC |
Not mentioned |
[54] |
|
LR3 |
GCAAGTCTGGTGCCAGCAGCC |
|||
|
25S |
LROR |
ACCCGCTGAACTTAAGC |
1431 |
[33] |
|
LR7 |
TACTACCACCAAGATCT |
|||
|
ACT |
ACT-512F |
ATGTGCAAGGCCGGTTTCGC |
300 |
[25] |
|
ACT-783R |
TACGAGTCCTTCTGGCCCAT |
|||
|
ß-tubulin |
Vd-btub-1F |
GCGACCTTAACCACCTCGTT |
Not mentioned |
[52] |
|
Vd-btub-1R |
CGCGGCTGGTCAGAGGA |
|||
|
VertBt-F |
AACAACAGTCCGATGGATAATTC |
Not mentioned |
[52] |
|
|
VertBt-R |
GTACCGGGCTCGAGATCG |
|||
|
VITubF2 |
GCAAAACCCTACCGGGTTATG |
143 |
[53] |
|
|
VITubRl |
AGATATCCATCGGACTGTTCGTA |
|||
|
VdTubF2 |
GGCCAGTGCGTAAGTTATTCT |
82 |
[53] |
|
|
VdTubR4 |
ATCTGGTTACCCTGTTCATCC |
|||
|
Bt2a |
GGTAACCAAATCGGTGCTGCTTTC |
Not mentioned |
[27] |
|
|
Bt2b |
ACCCTCAGTGTAGTGACCCTTGGC |
|||
|
CAL |
CL1 |
GARTWCAAGGAGGCCTTCTC |
Not mentioned |
[27] |
|
CL2 |
TTTTTGCATCATGAGTTGGAC |
|||
|
CAL-228F |
GAGTTCAAGGAGGCCTTCTCCC |
Not mentioned |
[45] |
|
|
CAL-737R |
CATCTTTCTGGCCATCATGG |
|||
|
EF-1α |
EF-1 |
ATGGGTAAGGAGGACAAGAC |
700 |
[37] |
|
EF-2 |
GGAGGTACCAGTGATCATGTT |
|||
|
EF1-728F |
CATCGAGAAGTTCGAGAAGG |
Not mentioned |
[45] |
|
|
EF2 |
GGAGGTACCAGTGATCATGTT |
|||
|
EF1-728F |
CATCGAGAAGTTCGAGAAGG |
350 |
[25] |
|
|
EF1-983R |
TACTTGAAGGAACCCTTACC |
|||
|
Endochitinase |
Vd-endoch-1F |
CTCGGAGGTGCCATGTACTG |
Not mentioned |
[52] |
|
Vd-endoch-1R |
ACTGCCTGGCCCAGGTTC |
|||
|
GAPDH |
Vd-G3PD-2F |
CACGGCGTCTTCAAGGGT |
Not mentioned |
[52] |
|
Vd-G3PD-1R |
CAGTGGACTCGACGACGTAC |
|||
|
GDF1 |
GCCGTCAACGACCCCTTCATTGA |
Not mentioned |
[27] |
|
|
GDR1 |
GGGTGGAGTCGTACTTGAGCATGT |
|||
|
gpd-1 |
CAACGGCTTCGGTCGCATTG |
Not mentioned |
[24] |
|
|
gpd-2 |
GCCAAGCAGTTGGTTGTGC |
|||
|
GS |
GSF1 |
ATGGCCGAGTACATCTGG |
Not mentioned |
[27] |
|
GSR1 |
GAACCGTCGAAGTTCCAC |
|||
|
ITS |
ITS1 |
TCCGTAGGTGAACCTGCGG |
334-738 |
|
|
ITS4 |
TCCTCCGCTTATTGATATGC |
|||
|
Vd-ITS1-45-F |
CCGGTCCATCAGTCTCTCTG |
334 |
[51] |
|
|
Vd-ITS2-379-R |
ACTCCGATGCGAGCTGTAAC |
|||
|
ITS1-F |
CTTGGTCATTTAGAGGAAGTAA |
700 |
[37] |
|
|
ITS4 |
TCCTCCGCTTATTGATATGC |
|||
|
VtF4 |
CCGGTGTTGGGGATCTACT |
123 |
[53] |
|
|
VtR2 |
GTAGGGGGTTTAGAGGCTG |
|||
|
ITS 4 |
TCCTCCGCTTATTGATATGC |
Not mentioned |
[27] |
|
|
ITS 5 |
GGAAGTAAAAGTCGTAACAAGG |
|||
|
RPB2 |
bRPB2-6F |
TGGGGYATGGTNTGYCCYGC |
Not mentioned |
[34] |
|
bRPB2-7R |
GAYTGRTTRTGRTCRGGGAAVGG |
|||
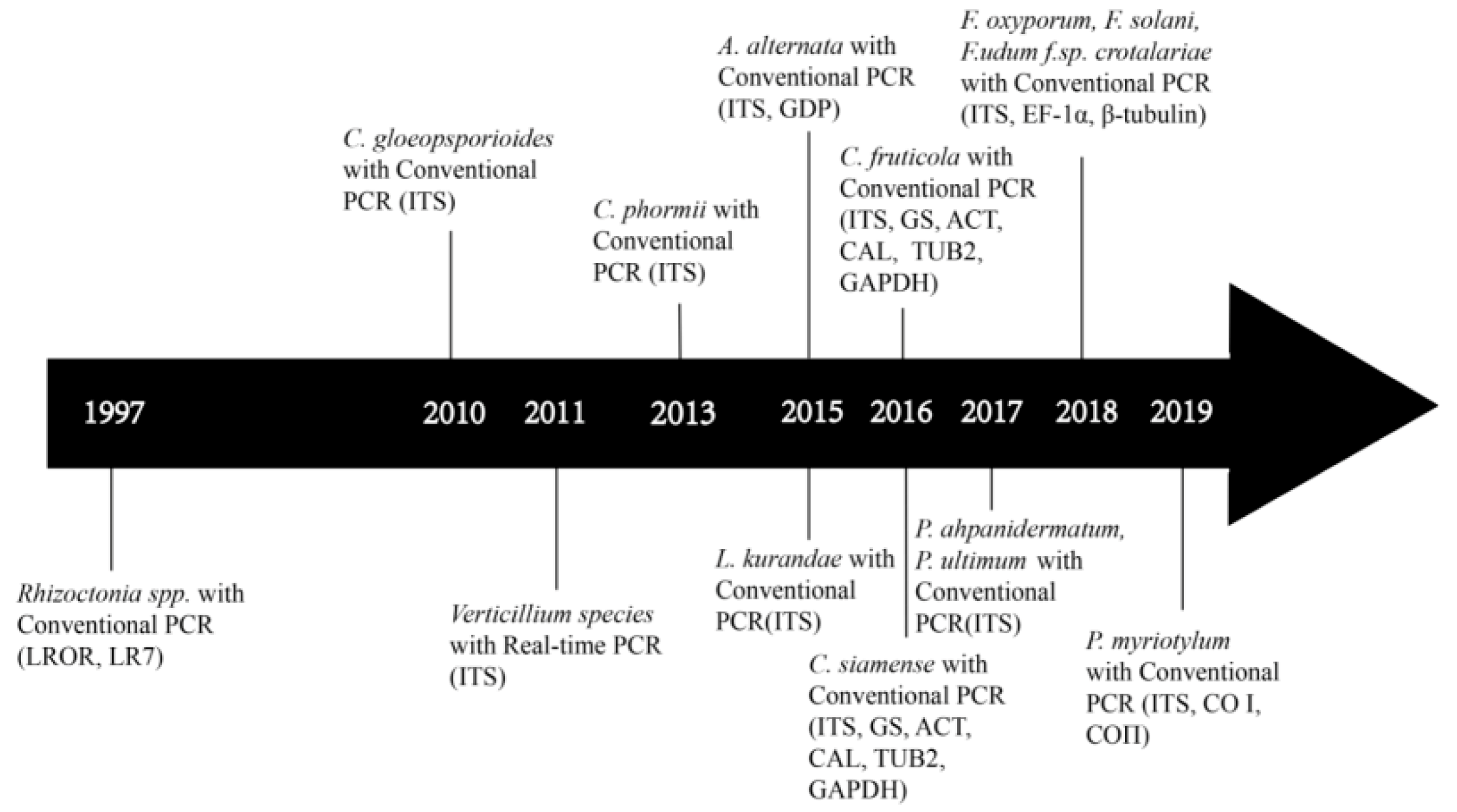
5. Target DNA Selection and Molecular Assays of Fungal Pathogens on Bast Fiber Crops
This entry is adapted from the peer-reviewed paper 10.3390/pathogens9030223
References
- Kang, Z.S. Current status and development strategy for research on plant fungal diseases in China. Plant Protect 2010, 36, 9–12. (In Chinese)
- Goyal, S.; Ramawat, K.G.; Mérillon, J.M. Different shades of fungal metabolites: An overview. In Fungal Metabolites; Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–29.
- Tsui, C.K.; Woodhall, J.; Chen, W.; Lévesque, C.A.; Lau, A.; Schoen, C.D.; Baschien, C.; Najafzadeh, M.J.; de Hoog, G.S. Molecular techniques for pathogen identification and fungus detection in the environment. IMA Fungus 2011, 2, 177–189.
- Guillemette, T.; Iacomi-Vasilescu, B.; Simoneau, P. Conventional and real-time PCR-based assay for detecting pathogenic Alternaria brassicae in cruciferous seed. Plant Dis. 2004, 88, 490–496.
- Lievens, B.; Brouwer, M.; Vanachter, A.C.R.C.; Cammue, B.P.A.; Thomma, B.P.H.J. Real-time PCR for detection and quantification of fungal and Oomycete tomato pathogens in plant and soil samples. Plant Sci. 2006, 171, 155–165.
- Luchi, N.; Capretti, P.; Pazzagli, M.; Pinzani, P. Powerful qPCR assays for the early detection of latent invaders: Interdisciplinary approaches in clinical cancer research and plant pathology. Appl. Microbiol. Biot. 2016, 100, 5189–5204.
- Wetzel, T.; Candresse, T.; Macquaire, G.; Ravelonandro, M.; Dunez, J. A highly sensitive immunocapture polymerase chain reaction method for plum pox potyvirus detection. J. Virol. Methods 1992, 39, 27–37.
- Khoodoo, M.H.R.; Sahin, F.; Jaufeerally-Fakim, Y. Sensitive detection of Xanthomonas axonopodispv. dieffenbachiae on Anthurium andreanum by immunocapture-PCR (IC-PCR) using primers designed from sequence characterized amplified regions (SCAR) of the blight pathogen. Eur. J. Plant Pathol. 2005, 112, 379–390.
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; (one of ~38 collaborators). High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011, 83, 8604–8610.
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63.
- Alka, R.; Nerida, D.; Nitin, M. Review: The future of plant pathogen diagnostics in a nursery production system. Biosens. Bioelectron. 2019, 145, 111631.
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169.
- Kuzdralinski, A.; Kot, A.; Szczerba, H.; Nowak, M.; Muszynska, M. A review of conventional PCR assays for the detection of selected phytopathogens of wheat. J. Mol. Microbiol. Biotechnol. 2017, 27, 175–189.
- Lau, H.Y.; Botella, J.R. Advanced DNA-based point-of-care diagnostic methods for plant diseases detection. Front. Plant Sci. 2017, 8, 2016.
- Tedersoo, L.; Drenkhan, R.; Anslan, S.; Morales-Rodriguez, C.; Cleary, M. High-throughput identification and diagnostics of pathogens and pests: Overview and practical recommendations. Mol. Ecol. Resour. 2019, 19, 47–76.
- McCartney, H.A.; Foster, S.J.; Fraaije, B.A.; Ward, E. Molecular diagnostics for fungal plant pathogens. Pest Manag. Sci. 2003, 59, 129–142.
- Young, B.A.; Hanson, K.E.; Gomez, C.A. Molecular diagnostic advances in transplant infectious diseases. Curr. Infect. Dis. Rep. 2019, 21, 52.
- Wickes, B.L.; Wiederhold, N.P. Molecular diagnostics in medical mycology. Nat. Commun. 2018, 9, 5135.
- Powers-Fletcher, M.V.; Hanson, K.E. Nonculture diagnostics in fungal disease. Infect. Dis. Clin. North Am. 2016, 30, 37–49.
- Pari, L.; Baraniecki, P.; Kaniewski, R.; Scarfone, A. Harvesting strategies of bast fiber crops in Europe and in China. Ind. Crops Prod. 2015, 68, 90–96.
- Fangueiro, R.; Rana, S. Natural Fibers: Advances in Science and Technology towards Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2010.
- Luo, S.Y.; Li, D.F.; Gong, Y.C.; Wang, Y.F.; Tang, S.W. Research on breeding of bast fiber crops of IBFC in the past 50 years. Plant Fiber Sci. China 2009, 31, 82–92. (In Chinese)
- Zeng, X.P.; Fu, M.Y.; He, S.; Wu, F.Z.; Wang, S.Y.; Chen, H.; Wang, H.F. Identification of the pathogen causing leaf spot disease on Cannabis sativa. Mol. Plant Breed. 2018, 16, 7094–7098. (In Chinese)
- Yu, Y.T.; Zeng, L.B.; Huang, L.l.; Yan, Z.; Sun, K.; Zhu, T.T.; Zhu, A.G. First report of black leaf spot caused by Alternaria alternata on ramie in China. J. Phytopathol. 2016, 164, 358–361.
- Doyle, V.P.; Tonry, H.T.; Amsden, B.; Beale, J.; Dixon, E.; Li, H.; Szarka, D.; Gauthier, N.W. First report of Cercospora cf. flagellaris on industrial hemp (Cannabis saliva) in Kentucky. Plant Dis. 2019, 103, 1784.
- Niu, X.P.; Gao, H.; Chen, Y.; Qi, J.M. First report of anthracnose on white jute (Corchorus capsularis) caused by Colletotrichum fructicola and C. siamense in China. Plant Dis. 2016, 100, 1243.
- Niu, X.; Gao, H.; Qi, J.; Chen, M.; Tao, A.; Xu, J.; Dai, Z.; Su, J. Colletotrichum species associated with jute (Corchorus capsularis L.) anthracnose in southeastern China. Sci. Rep. 2016, 6, 25179.
- Wang, X.X.; Wang, B.; Liu, J.L.; Chen, J.; Cui, X.P.; Jiang, H.; Peng, D.X. First report of anthracnose caused by Colletotrichum gloeosporioides on ramie in China. Plant Dis. 2010, 94, 1508.
- Wang, X.X.; Chen, J.; Wang, B.; Liu, L.J.; Huang, X.; Ye, S.T.; Peng, D.X. First report of anthracnose on Boehmeria nivea caused by Colletotrichum higginsianum in China. Plant Dis. 2011, 95, 1318.
- Serdani, M.; Rooney-Latham, S.; Wallis, K.M.; Blomquist, C.L. First report of Colletotrichum phormii causing anthracnose on New Zealand flax in the United States. Plant Dis. 2013, 97, 1115.
- Golzar, H.; Wang, C. First report of Colletotrichum phormii the cause of anthracnose on Phormium tenax in Australia. Aust. Plant Dis. Notes 2010, 5, 110–112.
- Kwon, J.H.; Lee, S.T.; Choi, Y.J.; Lee, S.D.; Kim, J. Outbreak of anthracnose on Hibiscus cannabinus caused by Colletotrichum sp in Korea. Plant Dis. 2015, 99, 1643–1644.
- McPartland, J.M.; Cubeta, M.A. New species, combinations, host associations and location records of fungi associated with hemp (Cannabis sativa). Mycol. Res. 1997, 101, 853–857.
- Thiessen, L.D.; Schappe, T. First report of Exserohilum rostratum causing foliar blight of industrial hemp (Cannabis saliva). Plant Dis. 2019, 103, 1414.
- Punja, Z.K.; Rodriguez, G. Fusarium and Pythium species infecting roots of hydroponically grown marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018, 40, 498–513.
- Ullah, M.W.; Haque, M.S.; Islam, M.S. First report of Fusarium oxysporum causing fusarium wilt on jute (Corchorus olitorius) in Bangladesh. Plant Dis. 2019, 103, 2673.
- Punja, Z.K.; Scott, C.; Chen, S. Root and crown rot pathogens causing wilt symptoms on field-grown marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018, 40, 528–541.
- Melo, M.P.; Beserra, J.J.E.A.; Matos, K.S.; Lima, C.S.; Pereira, O.L. First report of a new lineage in the Fusarium solani species complex causing root rot on sunn hemp in Brazil. Plant Dis. 2016, 100, 1784–1785.
- Wang, C.L.; Dai, Y.L. First report of sunn hemp fusarium wilt caused by Fusarium udum f. spcrotalariae in Taiwan. Plant Dis. 2018, 102, 1031.
- Szarka, D.; Tymon, L.; Amsden, B.; Dixon, E.; Judy, J.; Gauthier, N. First report of powdery mildew caused by Golovinomyces spadiceus on industrial hemp (Cannabis sativa) in Kentucky. Plant Dis. 2019, 103, 1773.
- Pépin, N.; Punja, Z.K.; Joly, D.L. Occurrence of powdery mildew caused by Golovinomyces cichoracearum sensu lato on Cannabis sativa in Canada. Plant Dis. 2018, 102, 2644.
- Gevens, A.J.; Maia, G.; Jordan, S.A. First report of powdery mildew caused fly Golovinomyces cichoracearum on Crotalaria juncea (‘Tropic Sun’ sunn hemp). Plant Dis. 2009, 93, 427.
- Norhayati, M.; Erneeza, M.H.; Kamaruzaman, S. Morphological, pathogenic and molecular characterization of Lasiodiplodia theobromae: A causal pathogen of black rot disease on kenaf seeds in Malaysia. Int. J. Agric. Biol. 2016, 18, 80–85.
- Choi, I.Y.; Kang, C.H.; Lee, G.H.; Park, J.H.; Shin, H.D. Sooty mould disease caused by Leptoxyphium kurandae on kenaf. Mycobiology 2015, 43, 347–350.
- Casano, S.; Hernandez, C.A.; Delgado, M.M.; Garcia-Tejero, I.F.; Gomez, S.O.; Puig, A.A.; Santos, B.D.L. First report of charcoal rot caused by Macrophomina phaseolina on hemp (Cannabis sativa) varieties cultivated in southern Spain. Plant Dis. 2018, 102, 1665–1666.
- Cho, S.E.; Zhao, T.T.; Choi, I.Y.; Choi, Y.J.; Shin, H.D. First report of powdery mildew caused by Podosphaera xanthii on ramie in Korea. Plant Dis. 2016, 100, 1495–1496.
- McGehee, C.S.; Apicella, P.; Raudales, R.; Berkowitz, G.; Ma, Y.; Durocher, S.; Lubell, J. First report of root rot and wilt caused by Pythium myriotylum on hemp (Cannabis sativa) in the United States. Plant Dis. 2019, 103, 3288.
- Beckerman, J.; Nisonson, H.; Albright, N.; Creswell, T. First report of Pythium aphanidermatum crown and root rot of industrial hemp in the United States. Plant Dis. 2017, 101, 1038–1039.
- Beckerman, J.; Stone, J.; Ruhl, G.; Creswell, T. First report of Pythium ultimum crown and root rot of industrial hemp in the United States. Plant Dis. 2018, 102, 2045.
- Koike, S.T.; Stangbellini, H.; Mauzey, S.J.; Burkhardt, A. First report of sclerotinia crown rot caused by Sclerotinia minor on hemp. Plant Dis. 2019, 103, 1771.
- Blum, A.; Bressan, M.; Zahid, A.; Trinsoutrot-Gattin, I.; Driouich, A.; Laval, K. Verticillium wilt on fiber flax: Symptoms and pathogen development in planta. Plant Dis. 2018, 102, 2421–2429.
- Bressan, M.; Blum, A.; Castel, L.; Trinsoutrot-Gattin, I.; Laval, K.; Gangneux, C. Assessment of Verticillium flax inoculum in agroecosystem soils using real-time PCR assay. Appl. Soil Ecol. 2016, 108, 176–186.
- Debode, J.; Van Poucke, K.; Franca, S.C.; Maes, M.; Höfte, M.; Heungens, K. Detection of multiple Verticillium species in soil using density flotation and real-time polymerase chain reaction. Plant Dis. 2011, 95, 1571–1580.
- Yu, Y.T.; Chen, J.; Gao, C.S.; Zeng, L.B.; Li, Z.M.; Zhu, T.T.; Sun, K.; Cheng, Y.; Sun, X.P.; Yan, L.; et al. First report of brown root rot caused by Pythium vexans on ramie in Hunan, China. Can. J. Plant Pathol. 2016, 38, 405–410.
- Prihastuti, H.; Cai, L.; Chen, H.; McKenzie, E.H.C.; Hyde, K.D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009, 39, 89–109.
- Laitinen, R.; Malinen, E.; Palva, A. PCR-ELISAI: Application to simultaneous analysis of mixed bacterial samples composed of intestinal species. Syst. Appl. Microbiol. 2002, 25, 241–248.
- Zhang, S.M.; Li, J.; Wang, Y.X.; Zhao, X.Y.; Zhang, X.C.; Meng, L.Q.; Tian, J.P. Advances in molecular diagnosis of plant fungal pathogens. J. Anhui Agric 2010, 38, 597–599, 664. (In Chinese)
- Bluhm, B.H.; Flaherty, J.E.; Cousin, M.A.; Woloshuk, C.P. Multiplex polymerase chain reaction assay for the differential detection of trichothecene-and fumonisin-producing species of Fusarium in Cornmeal. J. Food Protect. 2002, 65, 1955–1961.
- Demeke, T.; Clear, R.M.; Patrick, S.K.; Gaba, D. Species specific PCR-based assays for the detection of Fusarium species and a comparison with the whole seed agar plate method and trichothecene analysis. Int. J. Food. Microbiol. 2005, 103, 271–284.
- Torp, M.; Nirenberg, H.I. Fusarium langsethiae sp. nov. on cereals in Europe. Int. J. Food Microbiol. 2004, 95, 247–256.
- White, T.J.; Bruns, T.; Lee, S.; Tailor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322.
- Edwards, S.G.; O’Callaghan, J.; Dobson, A.D.W. PCR-based detection and quantification of mycotoxigenic fungi. Mycol. Res. 2002, 106, 1005–1025.
- Edel, V.; Steinberg, C.; Gautheron, N.; Alabouvette, C. Evaluation of restriction analysis of polymerase chain reaction (PCR)-amplified ribosomal DNA for the identification of Fusarium species. Mycol. Res. 1997, 101, 179–187.
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcode Consortium (one of ~100 collaborators). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246.
- Xu, J. Fungal DNA barcoding. Genome 2016, 59, 913–932.
- Hughes, K.W.; Petersen, R.H.; Lickey, E.B. Using heterozygosity to estimate a percentage DNA sequence similarity for environmental species’ delimitation across basidiomycete fungi. New Phytol. 2009, 182, 795–798.
