Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biophysics
|
Evolutionary Biology
The functionalized vesicle membrane is a product of the evolution process and is connected to several survival mechanisms. An octapeptide (KSPFPFAA) is identified which rapidly integrated into the vesicle membrane and is obviously connected to a particular advantage of the corresponding functionalized vesicle according to its significant accumulation.
- origin of life
- molecular dynamics
- aggregation process
- selection
- evolution
1. Introduction
In early prebiotic evolution, membranous boundaries were probably formed by simple amphiphilic molecules, easier to synthesize than phospholipids and able to integrate and accumulate other amphiphilic molecules. They would give rise to aggregation and membrane disruption, as well as to the formation of primitive membrane pores, thus enhancing selective molecular exchange between the first cell-like compartments and their environment [1][2].
A new hypothesis proposed by Schreiber et al. [3] proposes the early steps of the origin of life in deep-reaching tectonic faults. In this model, the formation of vesicular structures in these water- and CO2-filled zones has been proposed. The mechanism of formation includes cyclic phase transition processes between supercritical CO2 and subcritical gaseous CO2 by periodic pressure variations at a suitable depth [4]. In water/carbon dioxide interface zones, a pool of oligopeptides of variable length is generated by condensation reactions from amino acids in a hydrothermal environment [5]. Those oligopeptides, which have an amphiphilicity similar to the membrane of vesicular structures, will integrate and accumulate, increasing in concentration over time [6]. Based on the above model, Mayer et al. performed experiments with cyclic pressure variations at the water/CO2 interface, allowing vesicular structures to form from a mixture of long amine chains and long fatty acids previously proposed by Namani and Deamer [7]. Since the experiments were performed at 120 °C under acidic conditions, the octadecylamine/octadecanoic acid mixture was selected to improve the stability of the resulting vesicles. The continuous peptide accumulation combined with a selection of the vesicles for thermal stability led to an evolution-like process, finally resulting in functional peptides with the specific capability to make vesicles survive. One of the peptides that was particularly successful under these conditions turned out to be an octapeptide with the sequence H-Lys-Ser-Pro-Phe-Pro-Phe-Ala-Ala-OH (KSPFPFAA). In separate experiments, it could be shown that this peptide led to a reduced vesicle size, an increased membrane permeability, and to a significant increase in the thermal stability of the vesicles [8].
The main goal was to understand the particular mechanism of stabilization which has evolved in the pressure-cycling process. More explicitly, the entry wanted to elucidate the structural changes and interactions that are induced by the presence of the given octapeptide in the vesicle membrane. To this end, all-atom molecular dynamics were used to study the aggregation ability of the peptide KSPFPFAA in the octadecanoic acid/octadecylamine (1:1) bilayer membrane under conditions that mimic an acidic environment.
2. Cluster Process and Pore Formation
In order to obtain information about aggregation and pore formation of the KSPFPFAA peptide, the peptide–peptide and peptide–membrane interactions were studied. This was done in a parallel arrangement of sixteen peptides located perpendicular to the membrane surface, and in an antiparallel arrangement with alternating orientation of the peptides (Figure 1). The method described by Thøgersen et al. [9] was applied where the peptides are positioned at a starting distance of 2.8 nm, thus ensuring no prior interaction between adjacent peptides.
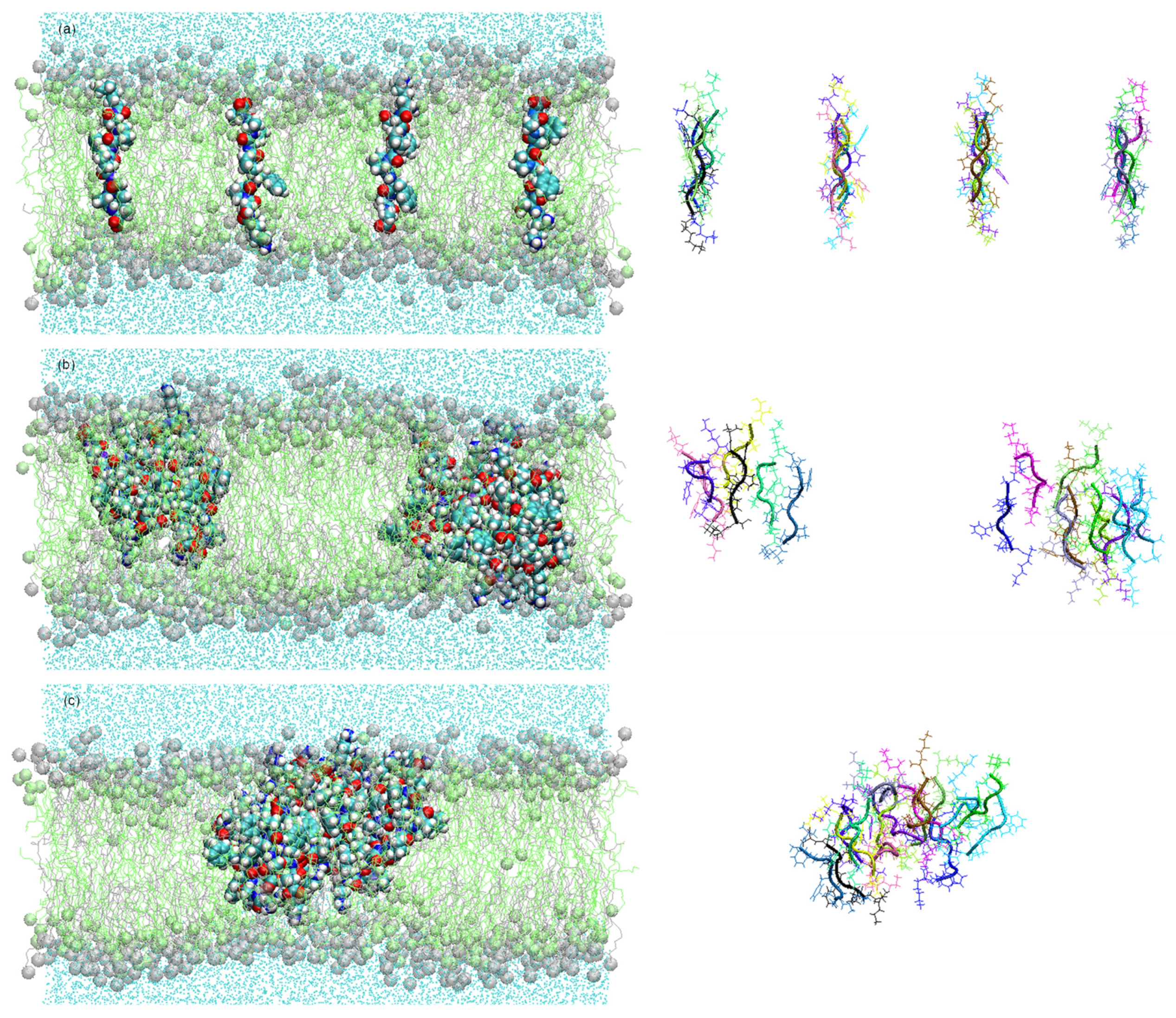
Figure 1. Snapshots of the simulation systems in the peptide aggregation process (peptides are shown as lines with cartoon representations on the right) in antiparallel arrangement at (a) 0 ns, (b) 100 ns, and (c) 450 ns.
Figure 2a shows the number of clusters formed in the antiparallel configuration during 450 ns MD simulation. The lateral diffusion of the octapeptide allows for rapid dimer formation in the first 8 ns and the stable cluster formation of up to five peptides at just 22 ns. Cluster size increases progressively and completes its total aggregation process at 428 ns.
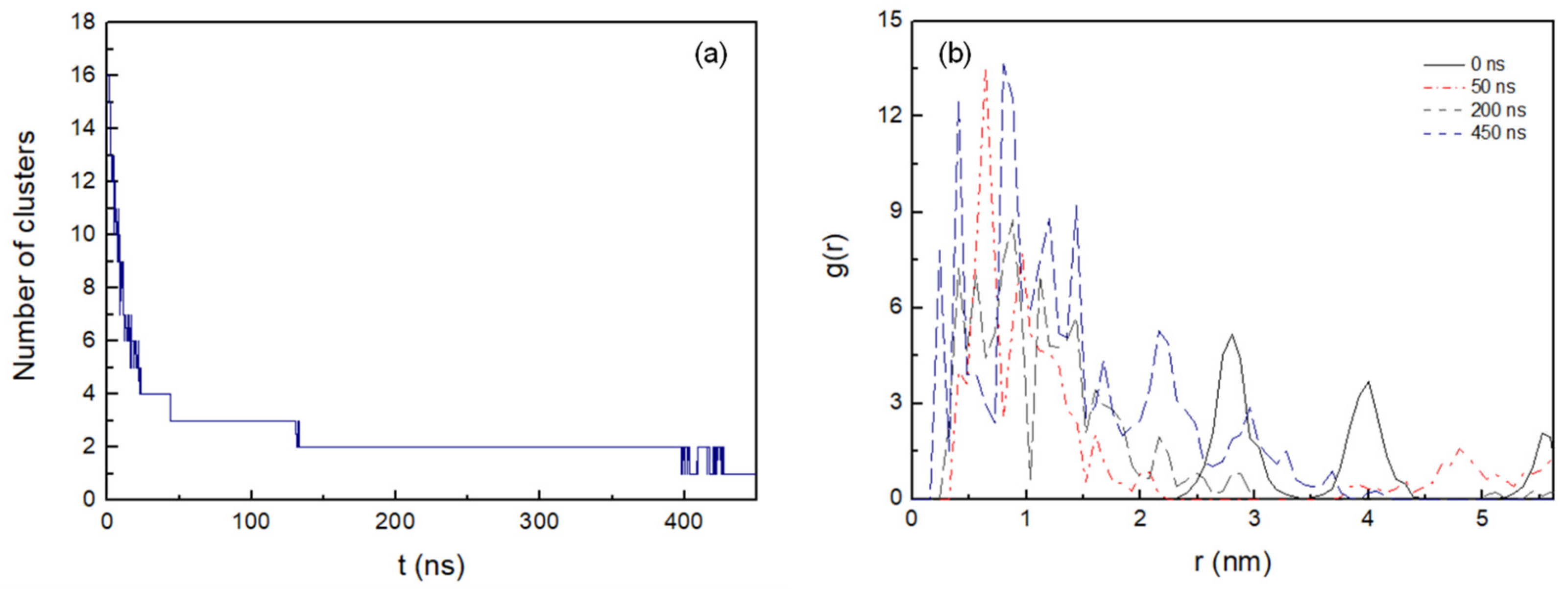
Figure 2. (a) Number of the clusters as a function of time formed during the 450 ns MD simulation with cut-off 0.6 nm. (b) Intermolecular radial distribution function, g(r), between the mass centres of the peptide pairings at 0 ns, 50 ns, 200 ns, and 450 ns (0.08 nm bin width).
The H-bonds between the peptide pairings were also determined, considering a donor-acceptor distance within 0.35 nm and 30° for the hydrogen-donor-acceptor angle (Figure 3a). The number of hydrogen bonds continuously increases during the simulation until stabilizing at around 175 ns.
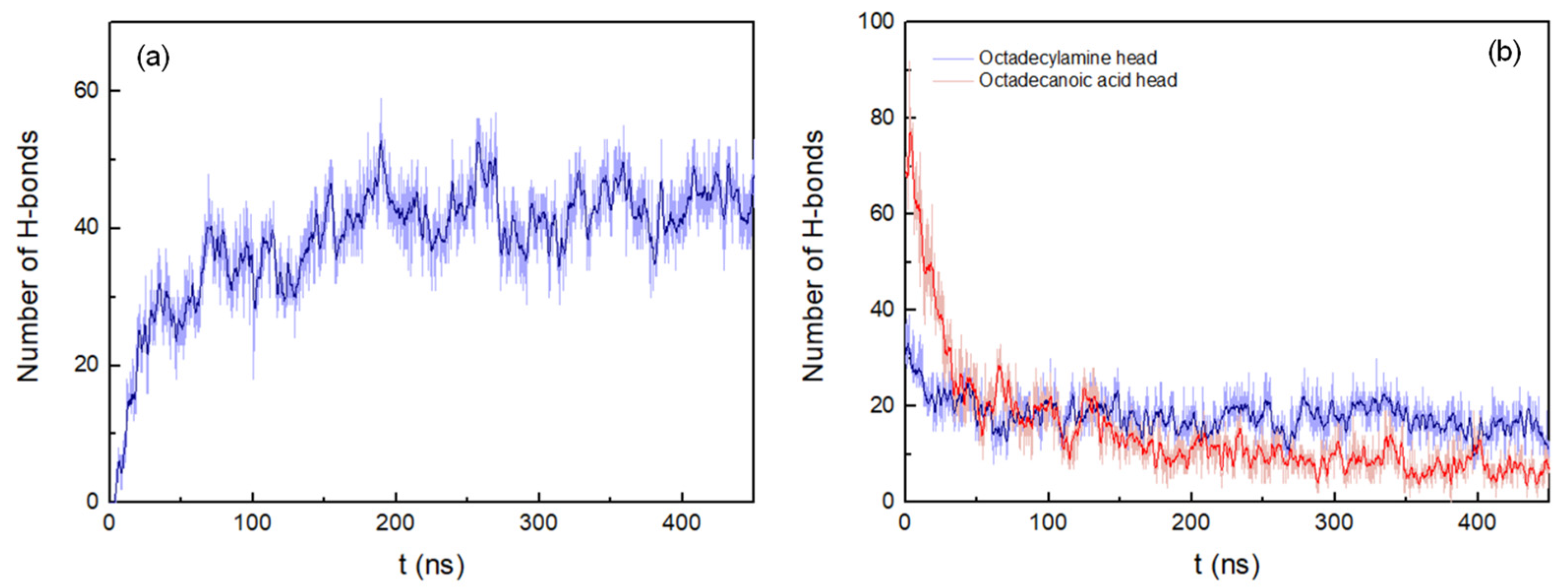
Figure 3. (a) Number of hydrogen bonds between peptides pairings. (b) Number of hydrogen bonds between octadecylamine and octadecanoic acid head groups and the peptides. Both representations show the results of the system with peptides of antiparallel arrangement up to 450 ns. A running average (window of length 2 ns) was applied to smooth the time series: (a), dark blue; (b), dark blue (octadecylamine head group–peptide interactions) and red (octadecanoic acid head group–peptide interactions).
The number of hydrogen bonds between the fatty acid head groups and the peptides in the antiparallel arrangement system decreases rapidly up to 54 ns. After that, the interactions deteriorate more slowly until approximately 350 ns. At this point in time, the number of hydrogen bonds begins to stabilize, corresponding to the end of the aggregation process (Figure 3b)
During the aggregation process, penetration of water molecules into the membrane was observed. Thus, in the pore formed by six peptides at 200 ns in the clustering process, MD simulations were carried out for another 100 ns to observe this effect more in detail (Figure 4).
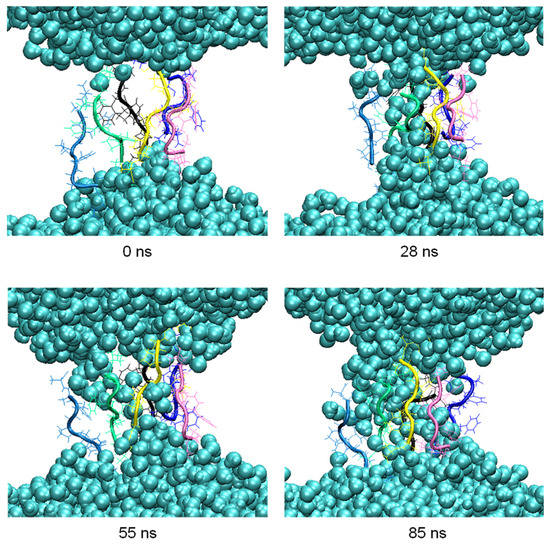
Figure 4. Snapshots of the penetration of the water molecules (blue spheres) into the membrane at relevant times of the 100 ns MD simulation.
The number of hydrogen bonds between the residues of the peptides forming the pore and the water molecules were analyzed in the 100 ns of simulation. The highest occurrences were observed in the terminal residues, K1 and A8, with 30% and 28%, respectively, and S2 with 18%. The difference in the occurrence of hydrogen bond formation between the residues of the octapeptide and the water molecules, and hence the ability of the pore to allow water molecules to flow inside the membrane, is influenced by the different orientations adopted by the side chains of the residues.
3. Research Findings
In the aggregation study, the peptides in an antiparallel arrangement remained embedded in the membrane throughout the process. Obviously, the antiparallel peptide arrangement leads to a significant stabilization via hydrogen bond interactions between the terminal sequences. Particularly, the intermolecular interaction between the amine hydrogen of K1 and the carbonyl oxygen of A8 gives rise to strong and stable trans-membrane aggregates. In addition, the polar head groups of octadecylamine and octadecanoic acid molecules tend to form hydrogen bonds with the A8 residue, which also contribute to the stabilization of the aggregates. Overall, it may also explain for the thermal stabilization of the overall membrane structure which has been observed experimentally by pulsed-field-gradient NMR [8].
In the same experiments, a distinct increase of the membrane permeability for water was observed. This phenomenon is reproduced by the given results from the molecular dynamics calculations. During the simulated aggregation process, it was possible to follow the peptide-induced penetration of water molecules into the membrane. Obviously, it is caused by the formation of central pores within peptide clusters in the membrane.
All in all, the simulation results clearly demonstrate why the octapeptide KSPFPFAA has a clear advantage against other peptide structures and therefore is accumulated in the experimental selection process. It forms a well-defined integration product with the bilayer membrane which is stabilizing the resulting composite structure thermodynamically. It enhances the membrane permeability for water, hereby relaxing the destructive osmotic pressure and leading to an increased survival rate of the corresponding vesicles.
This entry is adapted from the peer-reviewed paper 10.3390/life12020145
References
- Schrum, J.P.; Zhu, T.F.; Szostak, J.W. The origins of cellular life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002212.
- Deamer, D. The role of lipid membranes in life’s origin. Life 2017, 7, 5.
- Schreiber, U.; Locker-Grütjen, O.; Mayer, C. Hypothesis: Origin of life in deep-reaching tectonic faults. Orig. Life Evol. Biosph. 2012, 42, 47–54.
- Mayer, C.; Schreiber, U.; Dávila, M.J. Periodic vesicle formation in tectonic fault zones: An ideal scenario for molecular evolution. Orig. Life Evol. Biosph. 2015, 45, 139–148.
- Marshall, W.L. Hydrothermal synthesis of amino acids. Geochim. Cosmochim. Acta 1994, 58, 2099–2106.
- Mayer, C.; Schreiber, U.; Dávila, M.J. Selection of prebiotic molecules in amphiphilic environments. Life 2017, 7, 3.
- Namani, T.; Deamer, D.W. Stability of model membranes in extreme environments. Orig. Life Evol. Biosph. 2008, 38, 329–341.
- Mayer, C.; Schreiber, U.; Dávila, M.J.; Schmitz, O.J.; Bronja, A.; Meyer, M.; Klein, J.; Meckelmann, S.W. Molecular evolution in a peptide-vesicle system. Life 2018, 8, 16.
- Thøgersen, L.; Schiøt, B.; Vosegaard, T.; Nielsen, N.C.; Tajkhorshid, E. Peptide aggregation and pore formation in a lipid bilayer: A combined coarse-grained and all atom molecular dynamics study. Biophys. J. 2008, 95, 4337–4347.
This entry is offline, you can click here to edit this entry!
