Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Enterococcus has emerged as a significant nosocomial and community-acquired pathogen as a result of its ability to develop resistance to antimicrobials, particularly vancomycin. Vancomycin is the final treatment option, particularly for Enterococcus.
- vancomycin-resistant Enterococcus
- meta-analysis
- poultry
- Malaysia
1. Introduction
Human antimicrobial use, as well as their use as growth promoters in the livestock industry, were thought to have resulted in the emergence of enterococcal-resistant strains. A good example is the use of avoparcin as a feed additive to promote livestock growth [3].
The National Pharmaceuticals Regulatory Agency (NPRA) and the Department of Veterinary Services (DVS) in Malaysia have prohibited the use of avoparcin and vancomycin to reduce the spread or prevalence of vancomycin-resistant Enterococcus (VRE). DVS has been monitoring veterinary drug residues, including antibiotics, in animal feed since 2013, in accordance with EEC Directive 1990 [4]. This will invariably entail the monitoring of two antibiotic groups: group A, which includes banned substances such as avoparcin, chloramphenicol and vancomycin, and group B, which includes drugs with MRLs such as tetracycline. The most frequently used antibiotic classes in Malaysia are aminoglycosides, Beta-Lactams, microlides, tetracyclines, polymyxins, quinolones, sulfonamides and amphenicols [4]. What has brought VRE to the forefront in Malaysia is not only its critical public health concern but its potential economic impact on the livestock sector [3]. Antibiotic resistance poses great threat to food safety and public health when the resistant bacteria spread from food animals to poultry farmers, farmworkers and veterinarians through the food chain. As a result, antimicrobial resistance in poultry is a significant public health risk that warrants a discreet yet robust response. VRE has been reported in Malaysia amongst health workers, animals, hospital patients and farmworkers [3]. The epidemiology and transmission of resistant bacteria between humans and animals has increased, and their zoonotic potential cannot be underestimated [5].
2. The Pooled Prevalence of VRE in Poultry in Malaysia
The pooled prevalence of VRE in poultry in Malaysia was estimated at 24.0% (95% CI; 16.7–33.1%; I2 = 98.14%; p < 0.001) (Figure 1). Random-effects meta-analyses were carried out using the total sample size and number of positives (effect size, standard error of effect size) to estimate the prevalence of VRE in poultry in Malaysia. Between-study variability was high (t2 = 0.788; heterogeneity I2 = 98.14% with heterogeneity chi-square (Q) = 858.379, degrees of freedom (df) = 16, and p < 0.001). No individual study affected the heterogeneity and pooled prevalence of VRE in poultry in Malaysia as seen in the leave-one-out forest plot that was generated in the sensitivity analysis (Figure 2). More so, publication bias was observed as shown in the asymmetrical funnel plot (Figure 3). In addition to the funnel plots, the Trim-and-Fill method was then applied to include the “missing” studies from the analysis. The asymmetric studies were trimmed to locate the unbiased effect and fills the plot by re-inserting the trimmed studies as well as their imputed counterparts. Accordingly, four studies were missed and fell at the right side of the pooled estimate (Figure 4). In the Trim-and-Fill method, the adjusted estimate of VRE in poultry in Malaysia was 33.12% (95% CI; 24.3–43.3%). The Egger’s regression t = 1.777 (intercept = −4.0972; 95% CI; −0.75–2.57; p = 0.096) and Begg’s rank test (p = 0.30310) did not suggest significant publication bias.
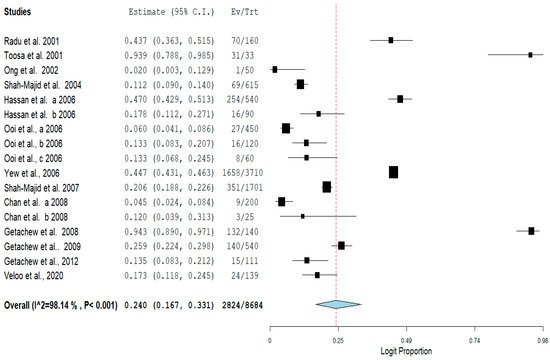
Figure 1. Forest plot showing the pooled prevalence of VRE in poultry in Malaysia.
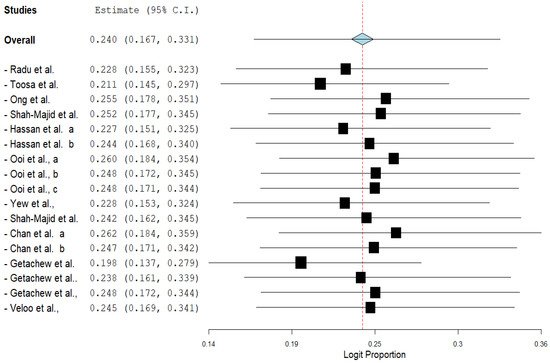
Figure 2. Leave-one-out forest plot of VRE in poultry in Malaysia.
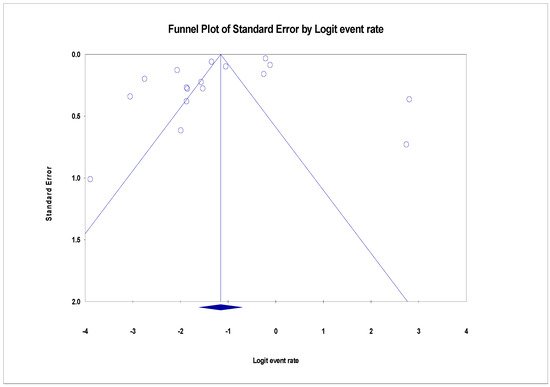
Figure 3. Funnel plot showing publication bias in studies reporting the prevalence of VRE in in poultry in Malaysia. Studies on the right side are fewer than those on the left and thus asymmetrical. The funnel plot is used for the visualization of bias.
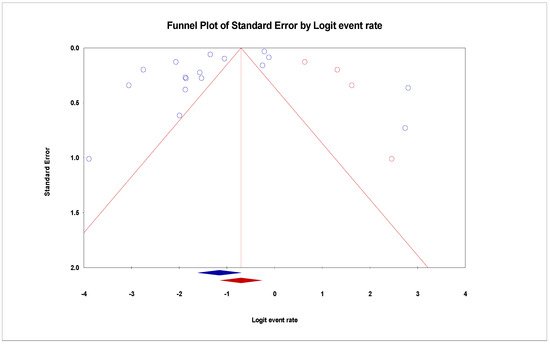
Figure 4. Funnel plot showing 4 added studies (in Red) in the Trim-and-Fill method reporting the prevalence of VRE in in poultry in Malaysia. This method simply looks for missing studies that will eventually eliminate bias. In this case, 4 studies will have to be added to the right side for the plot to be symmetrical.
3. Subgroup Meta-Analysis
To identify the possible sources of heterogeneity among studies, as substantial heterogeneity was observed, subgroup analysis was carried out using the year of study of the included studies, the regions where the studies were reported, the sources of VRE isolates and the method used in detecting VRE.
The result of subgroup meta-analysis by study year revealed overall large variability in studies reporting the prevalence of VRE (the Higgins I2 statistic = 98.14% with heterogeneity chi-square (Q) = 858.379, degrees of freedom = 16, and p < 0.001). However, most studies (n = 6) were reported in 2006 with only a single study reported as recently as 2018 and published in 2020. Studies carried out in 2005 (n = 2) and 2004 (n = 2) had a moderate (I2 = 56.15%) and highest (I2 = 99.49%) heterogeneity respectively. The forest plot for subgroup meta-analysis by study year is also shown in Figure 5.
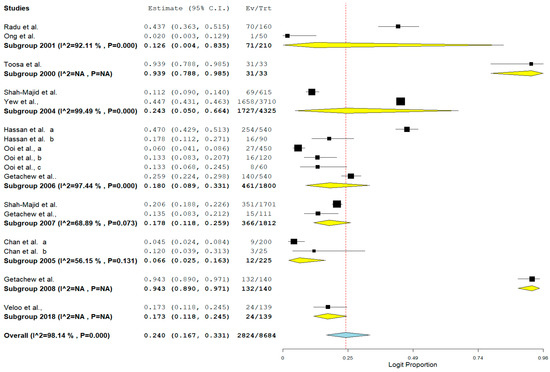
Figure 5. Forest plot showing the subgroup meta-analysis by study year of VRE in poultry in Malaysia.
The result of subgroup meta-analysis by study region showed that majority of the studies (n = 14) that reported the prevalence of VRE in poultry in Malaysia were from the Central region of Peninsular Malaysia with a prevalence of 29% and a high heterogeneity (I2 = 98.36%). The East coast region of the Malaysian Peninsular only had 2 studies with a prevalence of 6.6% and a moderate heterogeneity (I2 = 56.15%). In addition, the forest plot of subgroup meta-analysis by study region is shown in Figure 6.
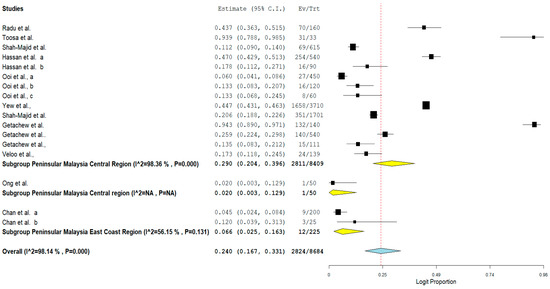
Figure 6. Forest plot showing the subgroup meta-analysis by study region of VRE in poultry in Malaysia.
Furthermore, the result of subgroup meta-analysis according to isolate sources revealed that most of the studies (n = 8) had their VRE isolated from chicken with a prevalence of 29.2% and a high heterogeneity (I2 = 98.61%). This was followed by 5 studies reporting the isolation of VRE from poultry environment with a prevalence of 12.5% and also a high heterogeneity (I2 = 81.07%). The forest plot is also shown in Figure 7.
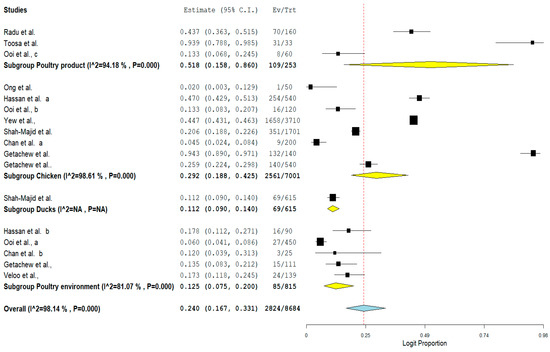
Figure 7. Forest plot showing the subgroup meta-analysis by Isolate sources of VRE in poultry in Malaysia.
Lastly, the result of subgroup meta-analysis according to the detection method showed that 5 studies each utilized disc diffusion and agar dilution with a prevalence of 45.6% and 23.1%, respectively. Only one study utilized polymerase chain reaction (PCR) as a method of detection with a prevalence of 43.7% and CI of 36.3–51.5%. The forest plot is shown in Figure 8.
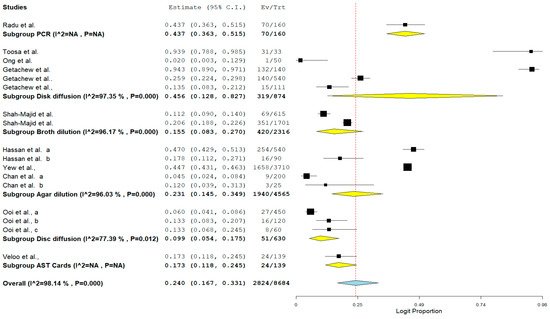
Figure 8. Forest plot showing the subgroup meta-analysis by detection method of VRE in poultry in Malaysia.
4. Meta-Regression
A separate meta-analysis was performed for each variable included and these are: the study year; the study region; the isolate source; and the detection method. A multivariate meta-regression analysis was utilized when the p-values of the variables in a single meta-regression is <0.25. In the final meta-analysis, all the variables listed were included. In the multivariate meta-regression, no analysis was recorded for studies carried out in 2007, 2008 and 2018. Most of the variables analyzed using multivariate meta-regression contributed to the heterogeneity observed in this study with a p-value of <0.05. Exceptions to these were studies whose isolates were from poultry products (p = 0.0873) and chicken (p = 0.520), study that utilized the use of AST cards as its detection methods (p = 0.427) and a study carried out in the year 2000 (p = 0.994).
5. Species Distribution of Enterococcus in Poultry in Malaysia
Nine species of Enterococcus were isolated in 11 studies reporting the prevalence of VRE in poultry in Malaysia, while 2 studies [13,18] did not report the species of Enterococcus isolated. Enterococcus faecalis was the most isolated (n = 563) and this was followed by E. faecium with 201 isolates.
6. Discussion
This is the first study to use meta-analysis and a systematic review to determine the prevalence of VRE in poultry in Malaysia, to the best of current knowledge. The pooled prevalence in this study is based on a thorough analysis of data from scientific publications on the prevalence of VRE in poultry in Malaysia published between 2001 and 2020. A meta-analysis was performed on 17 studies. The literature reviewed was heterogeneous, as expected, because the review included VRE reports from various regions, different study years, a variety of isolate sources and different methods for VRE detection. As a result, a random effect size model was used. This study’s high heterogeneity could be attributed to small-study effects and publication bias, because smaller studies sometimes show unusual, and often larger, treatment effects when compared to larger ones.
A small study with a larger-than-average impact is more likely to meet the statistical significance criterion, which may lead to an overestimation of true therapeutic effects. The assessment of publication bias is critical in meta-analysis. This is due to the fact that not all research findings are published, particularly those that are deemed unfavorable to a developed protocol or product, or those that would elicit only a minor amount of interest. Thus, studies that report relatively significant treatment effects are more likely to be submitted and/or approved for publication than studies that report more modest treatment effects. Current meta-analysis revealed a high level of variability, implying that the observed variability was compensated for by factors other than chance. The majority of the variables examined in these studies resulted in the observed heterogeneity. Similarly, studies with isolates from poultry products and chicken, studies with AST cards as detection methods and a study conducted in the year 2000 were not indicators of study heterogeneity.
There were no previous studies on meta-analysis to compare the prevalence of VRE in poultry in Malaysia, as this is the first study to analyze the prevalence of VRE in poultry in Malaysia. However, Wada et al. [1] reported a VRE pooled prevalence of 25% in Malaysia. In this current review, studies reporting the highest prevalence of VRE in poultry in Malaysia were mostly carried out in the Central region of the country. Most universities and research facilities such as the Department of Veterinary Services located in Putrajaya and National Pharmaceuticals Regulatory Agency (NPRA) which specializes in the surveillance of pathogens and regulations of drugs are located in the Central region of the country and this could be the reason why most of the studies were reported from that region. The density of poultry production in these regions could also be a factor. It is important that other regions are actively involved in the surveillance of Enterococcus and other pathogens, as this will help project the true estimate of the prevalence of VRE in poultry in Malaysia. Further, more up-to-date research is required as the most recent study reporting the prevalence of VRE in poultry was carried out in 2018 and published in 2020. It is either because the occurrence of resistant Enterococcus has reduced as a result of the ban on avoparcin or there is simply not enough surveillance going on to ascertain an actual estimate. Avoparcin and vancomycin were banned in Malaysia since 2013 by the National Pharmaceuticals Regulatory Agency (NPRA) and the Department of Veterinary Services (DVS) [4].
It is not surprising that most of the studies reported the isolation of VRE from chickens. Malaysians were expected to consume 48.7 kg of poultry meat per person in 2021, according to projections. This places Malaysia among the world’s top consumers of poultry meat [4]. In addition, the disc diffusion and agar dilution were the two most utilized VRE detection methods in poultry in Malaysia. Eight studies utilized the disc diffusion method, which has been reported to be straightforward and functional, with a well-standardized design, and it has the ability to provide categorical data that are simply understood by all practitioners, as well as the ability to choose from a variety of discs to test [19]. The lack of mechanization or automation of the disc test is one of its drawbacks. Only one study utilized PCR in the detection of VRE. For the detection and characterization of distinct VRE species, PCR is a quicker and more sensitive approach [20]. Finally, E. faecalis was the most isolated species of VRE in poultry in Malaysia from current analysis. The most frequent species capable of producing illness and creating an antibiotic resistance concern are E. faecalis and E. faecium, with E. faecalis accounting for the bulk of infections [21] E. faecalis is now recognized as a severe source of both hospital- and community-acquired urinary tract infections (UTIs), which can result in serious, life-threatening consequences such as bacteremia [22].
The use of antibiotics in animal husbandry varies by location and country. Antibiotics are sold in significantly larger quantities in low- and middle-income nations than they are in high-income countries [23]. As a region of fast-developing and integrated economies, Southeast Asia (SEA) is considered a hotspot location for antimicrobial resistance (AMR) [24]. Low- and middle-income nations utilized more antibiotics classified as medically important when compared to high-income countries [23]. The presence of veterinary drug traces in food samples confirmed this, indicating that violation rates in underdeveloped nations were higher than in developed nations [25].
SEA countries have had fast growth in the aquaculture and poultry production sectors in recent decades, accounting for a relatively large proportion of the worldwide veterinary antibiotic market [24]. Antibiotic management and therapy has become one of the most effective ways for these countries to avoid uncontrolled epidemic infections that could threaten their economies [24].
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics11020171
This entry is offline, you can click here to edit this entry!
