Corrosion is generally viewed as the destructive consequence of chemical reaction between a metal or metal alloy and its environment that contains corrosive agents. Compared to inorganic corrosion inhibitors, the organic ones are less toxic, can be also used at low concentrations, and have a better film-forming ability. The adsorption properties of organic corrosion inhibitors are primarily linked to the presence of both π-electrons from aromatic rings and heteroatoms in the molecular structures. Indeed, organic compounds containing nitrogen, phosphorus, sulfur, and oxygen atoms have been extensively investigated as corrosion inhibitors of metals and their alloys in acidic environments. In this respect, triazole derivatives are the most used nitrogen-containing organic inhibitors, in particular the family of 1,2,3-triazoles prepared under click chemistry regime by the prolific copper-catalyzed azide cycloaddition reactions.
- triazoles
- click chemistry
- corrosion inhibitor
- metal
- metal alloys
- mechanism of inhibition.
1. Synthesis of 1,2,3-triazoles
The [3+2] cycloaddition reaction between organic azides and alkynes catalyzed by copper(I) (CuAAC) is the most applicable and best known synthetic method for the selective preparation of 1,4-disubstituted-1,2,3-triazole derivatives. The first described preparative route to obtain triazole compounds was reported in the 1960s by Huisgen et al. [50]. This process requires high temperatures, typically solvent (toluene or carbon tetrachloride) reflux conditions, and prolonged reaction periods generally between 12 and 60 h. Under these thermal conditions, the two possible regioisomers (1,4- and 1,5-disubstituted-1,2,3-triazole derivatives) are formed in an equimolar proportion (top route in Figure 1). All this changed in 2002 when Sharpless, Fokin, and their colleagues at the Scripps Research Institute (at La Jolla) and Meldal’s group at the Carlsberg Laboratory (at Copenhagen) [21,51,52] worked in parallel on this reaction and independently discovered that copper(I) catalyzed the azide-alkyne cycloaddition reaction (CuAAC), providing a regioselectivity synthesis of only 1,4-disubstituted-1,2,3-triazoles in very high yields (bottom route in Figure 1).
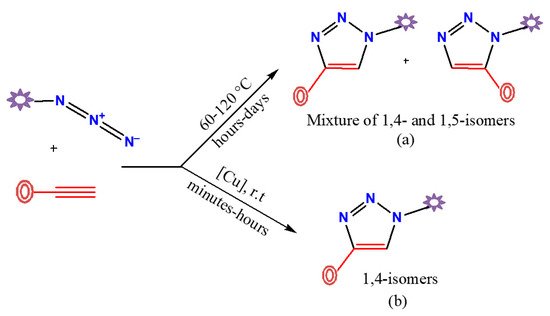
Figure 1. Non-regioselective (a) and regioselective (b) preparative routes of disubstituted-1,2,3-triazole derivatives by azide-alkyne cycloaddition reaction.
The continued research and development of new corrosion inhibitors is a matter of great importance and interest, both commercially and scientifically. Within this frame, several types of inhibitors have been developed and used to effectively inhibit the corrosion of metals; they can be classified as either inorganic or organic compounds. Typically, inorganic corrosion inhibitors have either anodic or cathodic action [73], while organic corrosion inhibitors have both anodic and cathodic roles (mixed-type), as well as a protective action by film adsorption (Figure 2). It is well established that the organic corrosion inhibitors are more effective and less expensive than the inorganic ones [74]. In this context, 1,2,3-triazole derivatives, which were initially studied as biologically active compounds and relevant medicinal compounds [55,56,75,76,77,78], have been reported as corrosion inhibitors for steels [79], copper, aluminum, and their alloys in corrosive media [80,81].
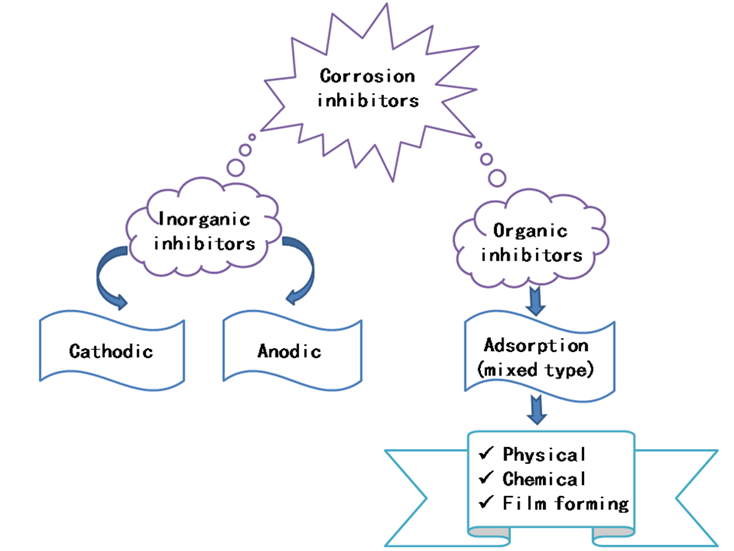
Figure 2. Classification of organic and inorganic corrosion inhibitors.
2. Mechanism of Corrosion Inhibition
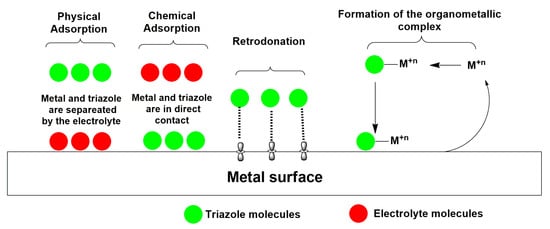
Figure 3. Adsorption behavior of the triazole inhibitors on the metal surface.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23010016
