Pulmonary arterial hypertension (PAH) is a rare, heterogeneous disease of the pulmonary vasculature, haemodynamically defined by a mean pulmonary arterial pressure (mPAP) >20 mmHg, a normal pulmonary artery wedge pressure ≤15 mmHg and elevated pulmonary vascular resistance ≥3 Wood units. Congenital heart disease (CHD) is frequently complicated by PAH, including four individual groups with shared features; Eisenmenger syndrome (ES), congenital systemic to pulmonary shunts, PAH associated with coincidental or small defects, and PAH encountered in patients with repaired congenital defects.
Spontaneous bleeding events are common in PAH-CHD and usually minor and self-limiting (e.g., dental bleeding, epistaxis, easy bruising, menorrhagia). Haemoptysis is one of the most perilous major bleeding manifestations in the clinical course of PAH-CHD and can be life-threatening.
- haemoptysis
- congenital heart disease
. Introduction
2. Pathophysiology of Haemoptysis
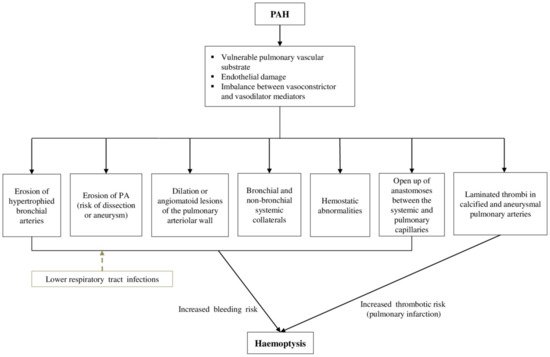
This entry is adapted from the peer-reviewed paper 10.3390/jcm11030633
References
- Gérald Simonneau; David Montani; David Celermajer; Christopher P. Denton; Michael A. Gatzoulis; Michael Krowka; Paul G. Williams; Rogerio Souza; Haemodynamic definitions and updated clinical classification of pulmonary hypertension. European Respiratory Journal 2019, 53, 1801913, 10.1183/13993003.01913-2018.
- Nazzareno Galiè; Marc Humbert; Jean-Luc Vachiery; Simon D J Gibbs; Irene Lang; Adam Torbicki; Gérald Simonneau; Andrew Peacock; Anton Vonk Noordegraaf; Maurice Beghetti; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal 2015, 37, 67-119, 10.1093/eurheartj/ehv317.
- Alexandra Arvanitaki; George Giannakoulas; Helmut Baumgartner; Astrid Elisabeth Lammers; Eisenmenger syndrome: diagnosis, prognosis and clinical management. Heart 2020, 106, 1638-1645, 10.1136/heartjnl-2020-316665.
- Alexandra Arvanitaki; Despoina Ntiloudi; George Giannakoulas; Konstantinos Dimopoulos; Prediction Models and Scores in Adult Congenital Heart Disease. Current Pharmaceutical Design 2021, 27, 1232-1244, 10.2174/1381612827999210111181554.
- Mark Schuuring; Annelieke C.M.J. van Riel; Jeroen C. Vis; Marielle G. Duffels; Arie van Dijk; Rianne H.A.C.M. de Bruin-Bon; Aeilko H. Zwinderman; Barbara J.M. Mulder; Berto Bouma; New predictors of mortality in adults with congenital heart disease and pulmonary hypertension: Midterm outcome of a prospective study. International Journal of Cardiology 2014, 181, 270-276, 10.1016/j.ijcard.2014.11.222.
- Despoina Ntiloudi; Sotiria Apostolopoulou; Konstantinos Vasiliadis; Alexandra Frogoudaki; Aphrodite Tzifa; Christos Ntellos; Styliani Brili; Athanasios Manginas; Antonios Pitsis; Marios Kolios; et al. Hospitalisations for heart failure predict mortality in pulmonary hypertension related to congenital heart disease. Heart 2018, 105, 465-469, 10.1136/heartjnl-2018-313613.
- Arabinda Saha; K.G. Balakrishnan; Pramod K. Jaiswal; C.G. Venkitachalam; Jaganmohan Tharakan; Thomas Titus; Raman Kutty; Prognosis for patients with Eisenmenger syndrome of various aetiology. International Journal of Cardiology 1994, 45, 199-207, 10.1016/0167-5273(94)90166-x.
- L. Daliento; J. Somerville; P. Presbitero; L. Menti; S. Brach-Prever; G. Rizzoli; S. Stone; Eisenmenger syndrome. Factors relating to deterioration and death. European Heart Journal 1998, 19, 1845-1855, 10.1053/euhj.1998.1046.
- Warren J Cantor; David A Harrison; Jack S Moussadji; Michael S Connelly; Gary D Webb; Peter Liu; Peter R McLaughlin; Samuel C Siu; Determinants of survival and length of survival in adults with Eisenmenger syndrome. The American Journal of Cardiology 1999, 84, 677-681, 10.1016/s0002-9149(99)00415-4.
- Craig S. Broberg; Masuo Ujita; Sanjay Prasad; Wei Li; Michael Rubens; Bridget E. Bax; Simon J. Davidson; Beatriz Bouzas; J. Simon R. Gibbs; John Burman; et al. Pulmonary Arterial Thrombosis in Eisenmenger Syndrome Is Associated With Biventricular Dysfunction and Decreased Pulmonary Flow Velocity. Journal of the American College of Cardiology 2007, 50, 634-642, 10.1016/j.jacc.2007.04.056.
- Sebastien Hascoet; Emmanuelle Fournier; Xavier Jais; Lauriane Le Gloan; Claire Dauphin; Ali Houeijeh; Francois Godart; Xavier Iriart; Adelaïde Richard; Jelena Radojevic; et al. Outcome of adults with Eisenmenger syndrome treated with drugs specific to pulmonary arterial hypertension: A French multicentre study. Archives of Cardiovascular Diseases 2017, 110, 303-316, 10.1016/j.acvd.2017.01.006.
- Cristel M. Sørensen Hjortshøj; Aleksander Kempny; Annette Jensen; Keld Sørensen; Edit Nagy; Mikael Dellborg; Bengt Johansson; Virginija Rudiene; Gu Hong; Alexander R. Opotowsky; et al. Past and current cause-specific mortality in Eisenmenger syndrome. European Heart Journal 2017, 38, 2060-2067, 10.1093/eurheartj/ehx201.
- Joo-Young Chun; Robert Morgan; Anna-Maria Belli; Radiological Management of Hemoptysis: A Comprehensive Review of Diagnostic Imaging and Bronchial Arterial Embolization. CardioVascular and Interventional Radiology 2010, 33, 240-250, 10.1007/s00270-009-9788-z.
- Lisa M. Tom; Harold I. Palevsky; Douglas S. Holsclaw; Scott O. Trerotola; Mandeep Dagli; Jeffrey I. Mondschein; S. William Stavropoulos; Michael C. Soulen; Timothy W.I. Clark; Recurrent Bleeding, Survival, and Longitudinal Pulmonary Function following Bronchial Artery Embolization for Hemoptysis in a U.S. Adult Population. Journal of Vascular and Interventional Radiology 2015, 26, 1806-1813.e1, 10.1016/j.jvir.2015.08.019.
- Bo Ram Lee; Jin Yeong Yu; Hee Jung Ban; In-Jae Oh; Kyu Sik Kim; Yong Soo Kwon; Yu Il Kim; Young Chul Kim; Sung Chul Lim; Analysis of Patients with Hemoptysis in a Tertiary Referral Hospital. Tuberculosis and Respiratory Diseases 2012, 73, 107-114, 10.4046/trd.2012.73.2.107.
- Marlene Rabinovitch; Pulmonary hypertension: pathophysiology as a basis for clinical decision making. The Journal of Heart and Lung Transplantation 1999, 18, 1041-1053, 10.1016/s1053-2498(99)00015-7.
- Gerhard-Paul Diller; Michael A. Gatzoulis; Pulmonary Vascular Disease in Adults With Congenital Heart Disease. Circulation 2007, 115, 1039-1050, 10.1161/circulationaha.105.592386.
- C. A. Wagenvoort; Vasoconstriction and Medial Hypertrophy in Pulmonary Hypertension. Circulation 1960, 22, 535-546, 10.1161/01.cir.22.4.535.
- Sheila G Haworth; PULMONARY HYPERTENSION IN THE YOUNG. Heart 2002, 88, 658-664, 10.1136/heart.88.6.658.
- Lulu M. Haroutunian; Catherine A. Neill; Pulmonary complications of congenital heart disease: Hemoptysis. American Heart Journal 1972, 84, 540-559, 10.1016/0002-8703(72)90479-6.
- Remy, J.; Remy‐Jardin, M.; Voisin, C.; Endovascular management of bronchial bleeding.. Lung Biol. Health Dis. 1992, 57, 667–723, .
- J Remy; L Lemaitre; J J Lafitte; M O Vilain; J Saint Michel; F Steenhouwer; Massive hemoptysis of pulmonary arterial origin: diagnosis and treatment. American Journal of Roentgenology 1984, 143, 963-969, 10.2214/ajr.143.5.963.
- Khalil, A.; Parrot, A.; Nedelcu, C.; Fartoukh, M.; Marsault, C.; Carette, M.F.; Severe hemoptysis of pulmonary arterial origin: Signs and role of multidetector row CT angiography. Chest 2008, 133, 212–219, .
- Darryl Tio; Edward Leter; Bart Boerrigter; Anco Boonstra; Anton Vonk-Noordegraaf; Harm Jan Bogaard; Risk Factors for Hemoptysis in Idiopathic and Hereditary Pulmonary Arterial Hypertension. PLOS ONE 2013, 8, e78132, 10.1371/journal.pone.0078132.
- T. J. Marshall; J. E. Jackson; Vascular intervention in the thorax: bronchial artery embolization for haemoptysis. European Radiology 1997, 7, 1221-1227, 10.1007/s003300050279.
- Nald M. McDonald; Angiogenesis and Remodeling of Airway Vasculature in Chronic Inflammation. American Journal of Respiratory and Critical Care Medicine 2001, 164, S39-S45, 10.1164/ajrccm.164.supplement_2.2106065.
- M E Deffebach; N B Charan; S Lakshminarayan; J Butler; The bronchial circulation. Small, but a vital attribute of the lung.. American Review of Respiratory Disease 1987, 135, 463–481, 10.1164/arrd.1987.135.2.463.
- Pump, K.K.; The Bronchial Arteries and their Anastomoses in the Human Lung. JAMA 1963, 184, 225, 10.1001/jama.1963.03700140159103.
- P Henriksson; G Varendh; N R Lundstrom; Haemostatic defects in cyanotic congenital heart disease.. Heart 1979, 41, 23-27, 10.1136/hrt.41.1.23.
- Sarah K. Westbury; Kurtis Lee; Christopher Reilly-Stitt; Robert Tulloh; Andrew D. Mumford; High haematocrit in cyanotic congenital heart disease affects how fibrinogen activity is determined by rotational thromboelastometry. Thrombosis Research 2013, 132, e145-e151, 10.1016/j.thromres.2013.07.006.
- A.S. Jensen; P.I. Johansson; L. Bochsen; L. Idorn; K.E. Sørensen; U. Thilén; E. Nagy; E. Furenäs; L. Søndergaard; Fibrinogen function is impaired in whole blood from patients with cyanotic congenital heart disease. International Journal of Cardiology 2013, 167, 2210-2214, 10.1016/j.ijcard.2012.06.019.
- Michael C. Lill; Joseph K. Perloff; John S. Child; Pathogenesis of Thrombocytopenia in Cyanotic Congenital Heart Disease. The American Journal of Cardiology 2006, 98, 254-258, 10.1016/j.amjcard.2006.01.083.
- Barbara LeVarge; Prostanoid therapies in the management of pulmonary arterial hypertension. Therapeutics and Clinical Risk Management 2015, 11, 535-547, 10.2147/tcrm.s75122.
- Hossein-Ardeschir Ghofrani; Miguel-Angel Gomez Sanchez; Marc Humbert; David Pittrow; Gérald Simonneau; Henning Gall; Ekkehard Grünig; Hans Klose; Michael Halank; David Langleben; et al. Riociguat treatment in patients with chronic thromboembolic pulmonary hypertension: Final safety data from the EXPERT registry. Respiratory Medicine 2020, 178, 106220, 10.1016/j.rmed.2020.106220.
- Paul Wood; The eisenmenger syndrome: Or pulmonary hypertension week reversed central shunt. The American Journal of Cardiology 1972, 30, 172-174, 10.1016/0002-9149(72)90203-2.
