White meat is the nutritional term for lighter-colored meat that contains less myoglobin than red meat, which contains a great deal. White meat includes poultry (e.g., chicken, duck, goose and turkey), fish, reptiles (e.g., land snail), amphibians (e.g., frog), crustaceans (e.g., shrimp and crab) and bivalves (e.g., oyster and clam), but it excludes all mammal flesh such as beef, pork, and lamb. White meat has high nutritional value and plays an important role in human diet. The production and sale of white meat need to meet specific quality and safety standards. Fluorescence spectroscopy, color imaging and multispectral imaging (MSI) have emerged as effective analytical methods for the non-destructive detection of quality attributes of various white meat products such as fish, shrimp, chicken, duck and goose.
- white meat
- multispectral imaging
- fluorescence spectroscopy
- convolutional neural network
- quality detection
1. Introduction
|
Technology |
Product |
Target Attributes |
Reference |
|---|---|---|---|
|
MSI |
Meat |
Adulteration |
Ropodi et al. [12] |
|
MSI, HSI |
Meat |
Defects |
Feng et al. [13] |
|
MSI |
Food |
Quality |
Su and Sun [14] |
|
MSI, IRS, SERS, LIBS and HSI |
Food |
Quality |
Wang et al. [15] |
|
MSI, HSI and VS |
Food |
Authenticity, quality and safety |
Ropodi et al. [16] |
|
Fluorescence spectroscopy |
Food |
Quality |
Karoui and Blecker [17] |
|
Fluorescence spectroscopy |
Food |
Quality |
Strasburg and Ludescher [18] |
|
Visible/Infrared, Raman and Fluorescence spectroscopy |
Raw and processed food |
Quality |
He and Sun [19] |
|
Fluorescence spectroscopy |
Food |
Quality |
Ahmad et al. [20] |
|
Fluorescence spectroscopy |
Dairy products |
Quality and safety |
Shaikh and O’Donnell [21] |
|
Fluorescence spectroscopy |
Fresh and frozen-thawed muscle foods |
Muscle classification |
Hassoun [22] |
|
RGB-Imaging |
Meat |
Quality and safety |
Taheri-Garavand et al. [23] |
|
RGB-Imaging |
Fish |
Quality |
Dowlati et al. [24] |
|
RGB-Imaging |
Food |
Quality |
Gomes and Leta [25] |
|
RGB-Imaging |
Food |
Quality |
Amani et al. [26] |
MSI––Multispectral imaging; HSI––Hyperspectral imaging; IRS––Infrared spectroscopy; SERS––Surface-Enhanced Raman Spectroscopy; LIBS––Laser induced breakdown spectroscopy; VS––Vibrational Spectroscopy.
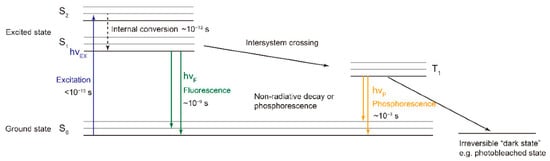
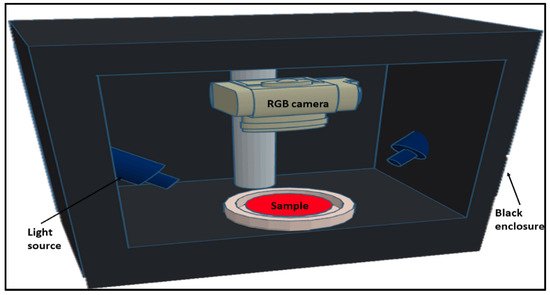
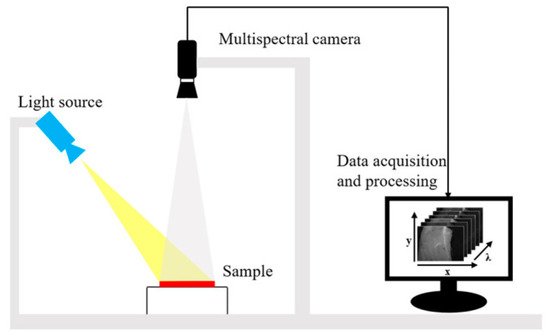
2. Fluorescence Spectroscopy, RGB- and Multispectral-Imaging
3. Quality Evaluation of White Meat
|
White Meat |
Module |
Quality Parameters |
Accuracy |
Reference |
|---|---|---|---|---|
|
Fish |
MSI |
TVB-N, PPC |
R2p = 0.862 for TVB-N, R2p = 0.921 for PPC |
Khoshnoudi-Nia and Moosavi-Nasab [39], Khoshnoudi-Nia and Moosavi-Nasab [40] |
|
Fish |
MSI |
TVC |
R2 = 0.62 |
Govari, et al. [41] |
|
Fish |
MSI |
TVC |
R2 = 0.683 |
Fengou, et al. [42] |
|
Fish |
MSI |
Astaxanthin concentration |
R2 = 0.86 |
Dissing, et al. [43] |
|
Fish |
MSI |
TVB-N, TBARS, K |
R2p = 0.922 for TVB-N, R2p = 0.867 for TBARS, R2p = 0.936 for K |
Cheng, et al. [44] |
|
Fish |
MSI |
A ‘standard freshness index’ of K |
R2 = 0.94, |
Omwange, et al. [45] |
|
Fish |
Fluorescence spectroscopy |
A ‘standard freshness index’ of K |
R2 = 0.92 |
Omwange, et al. [46] |
|
Fish |
Fluorescence spectroscopy |
A ‘standard freshness index’ of K |
R2 = 0.95 |
Liao, et al. [47] |
|
Fish |
Fluorescence spectroscopy |
AEC; NADH |
R2 = 0.90 for AEC, R2 = 0.85 for NADH |
Rahman, et al. [48] |
|
Fish |
Fluorescence spectroscopy |
NADH |
90.5% |
Hassoun and Karoui [49] |
|
Fish |
RGB imaging |
Classification performance |
99.5% |
Park, et al. [50] |
|
Fish |
RGB imaging |
Astaxanthin concentration |
R2 = 0.66 |
Dissing et al. [43] |
|
Fish |
RGB imaging |
Freshness of tuna meat cuts |
86.67% |
Lugatiman, et al. [51] |
|
Fish |
RGB imaging |
The main color of the sample |
75% |
Mateo, et al. [52] |
|
Fish |
RGB imaging |
Texture features |
86.3% |
Gu, et al. [53] |
|
Fish |
RGB imaging |
Color of Salmon Fillets |
R = 0.95 |
Quevedo, et al. [54] |
|
Fish |
RGB imaging |
Gill and eye color changes in the sparus aurata |
R2 = 0.994 |
Dowlati, et al. [55] |
|
Fish |
RGB imaging |
Body color of carp |
94.97% |
Taheri-Garavand, et al. [56] |
|
Fish |
RGB imaging |
Freshness |
98.2% |
Rocculi, et al. [57] |
|
Shrimp |
Fluorescence spectroscopy |
4-hexylresorcinol |
81.6% |
Jonker and Dekker [58] |
|
Shrimp |
Fluorescence spectroscopy |
K, pH |
R2 = 0.80 |
Rahman, et al. [59] |
|
Shrimp |
RGB imaging |
pH |
100% |
Witjaksono, et al. [60] |
|
Shrimp |
RGB imaging |
Identification accuracy of the proposed ShrimpNet for shrimp |
95.48% |
Hu, et al. [61] |
|
Shrimp |
RGB imaging |
Shrimp dehydration levels |
R = 0.86 |
Mohebbi, et al. [62] |
|
Shrimp |
RGB imaging |
Color changes in the head, legs and tail of pacific white shrimp (litopenaeus vannamei) |
90% |
Ghasemi-Varnamkhasti, et al. [63] |
|
Chicken |
Fluorescence spectroscopy |
Hydroxyproline concentration |
R2 = 0.82 |
Monago-Maraña, et al. [64] |
|
Chicken |
MSI |
Skin tumors |
86% |
Chao, et al. [65] |
|
Chicken |
MSI |
TVC |
90.4% |
Spyrelli, et al. [66] |
|
Chicken |
MSI |
pork-chicken adulteration |
90.00% for fresh samples, 86.67% for frozen-thawed samples |
Fengou, et al. [67] |
|
Chicken |
MSI |
Sepsis in chickens |
98.6% for septic chickens, 96.3% for healthy chickens |
Yang, et al. [68] |
|
Chicken |
MSI |
Contamination detection |
96% |
Park, et al. [69] |
|
Chicken |
MSI |
Chicken heart disease characterization |
100% |
Chao, et al. [70] |
|
Chicken |
MSI; Fluorescence spectroscopy |
Contamination detection |
92.5% |
Seo, et al. [71] |
|
Chicken |
Fluorescence spectroscopy |
Lipid oxidation |
R = 0.73 |
Gatellier, et al. [72] |
|
Chicken |
Fluorescence spectroscopy |
P. aeruginosa concentration |
96% |
Abdel-Salam, et al. [73] |
|
Chicken |
Fluorescence spectroscopy |
chicken meat tenderness |
R = 0.870 |
Yu, et al. [74] |
|
Chicken |
Fluorescence spectroscopy |
Contamination detection |
96.6% |
Cho, et al. [75] |
|
Chicken |
Fluorescence spectroscopy |
Measurement of lipid oxidation |
98% |
Wold and Kvaal [76] |
|
Chicken |
RGB imaging |
Avian flu infected chickens |
97.43% |
Cuan, et al. [77] |
|
Chicken |
RGB im-aging |
Color |
94% |
Yumono, et al. [78] |
|
Chicken |
RGB im-aging |
Freshness |
R = 0.987 |
Taheri-Garavand, et al. [79] |
|
Duck |
Fluorescence spectroscopy |
Gentamicin Residual in Duck Meat |
R = 0.996 |
Wang, et al. [80] |
|
Duck |
Fluorescence spectroscopy |
Doxycycline content in duck meat |
R = 0.998 |
Wang, et al. [81] |
|
Duck |
Fluorescence spectroscopy |
Carbaryl residue in duck meat |
R = 0.976 |
Xiao et al. [10] |
|
Duck |
Fluorescence spectroscopy |
Tetracycline content |
R = 0.952 |
Zhao, et al. [82] |
|
Duck |
Fluorescence spectroscopy |
Triazophos content |
R2p = 0.974, |
Zhao, et al. [83] |
|
Duck |
Fluorescence spectroscopy |
Neomycin residue |
R = 0.999 |
Jiang, et al. [84] |
|
Duck |
Fluorescence spectroscopy |
Carbofuran residue |
R2p = 0.999 |
XIAO, et al. [85] |
This entry is adapted from the peer-reviewed paper 10.3390/bios12020076
