Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Cilia are microtubule-based hair-like organelles on the cell surface. Cilia have been implicated in various biological processes ranging from mechanosensation to fluid movement. Ciliary dysfunction leads to a plethora of human diseases, known as ciliopathies.
- cilia
- multiciliated cells
- efferent ducts
- spermatogenesis
1. Introduction
Cilia are microtubule-based, tiny hair-like organelles that extend from the apical surface of many different cell types [1][2][3]. Extensive work over the last few decades has revealed that cilia regulate a wide range of biological processes during development and adult homeostasis. Cilia are broadly classified into two types according to their microtubule composition: non-motile primary cilia with a 9 + 0 microtubule arrangement and motile multicilia with a 9 + 2 arrangement. The primary cilia in the embryonic node are a notable exception to this rule [4][5]. Nodal cilia, which are responsible for the establishment of the left–right body axis, have a 9 + 0 microtubule arrangement but show motility. The ciliary axoneme, which is composed of microtubule bundles with associated proteins, is surrounded by a specialized ciliary membrane that is continuous with the plasma membrane but has distinct properties with unique lipid and receptor compositions [6][7]. Primary cilia are present on many different cell types in the human body and play key roles in mechanosensation, photoreception, and various intracellular signaling pathways, including hedgehog (Hh), platelet-derived growth factor (PDGF), and G-protein-coupled receptor (GPCR) pathways [1][2]. In contrast, multicilia are found in limited locations in the human body, such as epithelial cells lining the respiratory tract, the female and male reproductive tracts, and the brain ventricles. Multiciliated cells (MCCs) contain dozens to hundreds of motile cilia that beat in a whip-like motion. The synchronous motility of multicilia is achieved by dynein motors in an ATP-dependent manner. The force generated by the ciliary beating typically drives directional fluid flow, for example, to clear airway mucus and debris, transport ova from the ovary to the uterus [8], and circulate cerebrospinal fluid in the brain [9]. Sperm flagella are more complex, singular motile cilia that are important for swimming and fertilization [10][11].
Cilia are assembled from mother centriole-derived basal bodies. In contrast to the daughter centriole, the mother centriole harbors accessory structures, including subdistal and distal appendages. The distal appendages (also called “transition fibers” at the ciliary base) are critical for the recruitment of small vesicles and subsequent docking of basal bodies to the plasma membrane [12][13][14][15]. Most ciliary proteins are imported from the cell body via polarized vesicle trafficking from the Golgi complex or endosomes [16][17]. The extension of a cilium and its maintenance require intraflagellar transport (IFT), a bidirectional transport system that tracks along the axonemal microtubules [18][19]. Although the mode of centriole generation differs, the formation of both primary cilia and multicilia follows a similar pathway as discussed in more detail below [20][21].
Given the ubiquitous presence of cilia and their important roles in embryonic development and adult homeostasis, it is not surprising that genetic defects in the structure and function of cilia are associated with a host of human disorders, collectively known as ciliopathies [2][3][22]. Dysfunctional primary cilia have been linked to various diseases and syndromes, such as polycystic kidney disease (PKD), Joubert syndrome (JBTS), and Bardet–Biedl syndrome (BBS) [3][23][24]. Their clinical features are variable but include situs inversus, polydactyly, retinal degeneration, intellectual disability, obesity, and cystic lesions of the kidney, liver, and pancreas. On the other hand, defects in multicilia are most prominently associated with primary ciliary dyskinesia (PCD) [25][26][27][28]. PCD is a rare genetic disorder, affecting approximately 1:10,000–40,000 individuals. To date, over 50 causative genes have been reported, including various axonemal components and regulatory factors of axonemal assembly and MCC differentiation [11][27][29]. PCD is characterized by recurrent respiratory infections, situs inversus, infertility, and more rarely hydrocephalus. Male infertility and sub-fertility are also common manifestations of PCD.
2. Differentiation of Multiciliated Cells (MCCs)
MCC differentiation is orchestrated by a coordinated cascade of regulators, including signaling pathways and transcription factors (Figure 1). Previous studies have demonstrated that the inhibition of the Notch and bone morphogenic protein (BMP) signaling pathways triggers a regulatory network of genes involved in the MCC fate determination of developing epithelia [30][31][32][33]. The upstream mechanisms that repress the Notch and BMP pathways are not fully understood. However, the microRNA clusters miR-34/miR-449 have been shown to play essential roles at multiple levels in multiciliogenesis by destabilizing target mRNAs [34][35]. The miR-34/miR-449 family includes the functionally redundant miRNA clusters miR-34b/c and miR-449a/b/c, which are highly expressed in multiciliated tissues. In particular, miR-449 has been shown to promote MCC differentiation by directly repressing Notch1 and its ligand Delta-like 1 (DLL1) in progenitor cells [35][36][37].
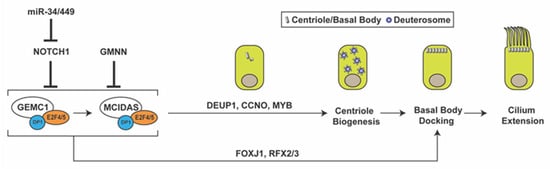
Figure 1. Multiciliated cell differentiation. Key factors and events for MCC differentiation are depicted. Repression of Notch signaling by miR-34/miR-449 triggers MCC differentiation. GEMC1 acts in a complex with E2F4/5 and DP1 to turn on MCIDAS and other ciliary genes necessary for centriole amplification, basal body docking, and cilium elongation. GMNN functions as an inhibitor of GEMC1 and MCIDAS. GMNN, geminin; GEMC1, geminin coiled-coil domain-containing protein 1; DEUP1, deuterosome assembly protein 1; CCNO, cyclin O; FOXJ1, forkhead box J1.
Downstream of Notch inhibition, the MCC differentiation gene regulatory network is controlled by the coiled-coil Geminin family proteins Geminin (GMNN), Geminin coiled-coil domain-containing protein 1 (GEMC1), and MCIDAS (also known as multicilin or IDAS) [38][39][40][41][42][43][44][45][46]. Both GEMC1 and MCIDAS are master regulatory proteins that are necessary and sufficient for MCC differentiation [43][47]. Upon forming a ternary complex with the cofactor DP1 and transcription factors E2F4/5, GEMC1 is able to activate expression of MCIDAS and other downstream ciliary genes. MCIDAS also acts as a transcriptional activator by forming a complex with DP1 and E2F4/5 to stimulate gene expression essential for centriole biogenesis. Early multiciliogenesis factors that are downstream of GEMC1 and MCIDAS include deuterosome assembly protein 1 (DEUP1), MYB, cyclin O (CCNO), forkhead box J1 (FOXJ1), and RFX2/3 [41][43][44][45][48]. p73, another transcription factor downstream of MCIDAS, was found to activate expression of FOXJ1, RFX2/3, and miR-34b/c and nearly 50 other ciliary genes in mouse tracheal MCCs [49][50]. While the role of Geminin in MCC differentiation is less understood, it has been shown to act as an inhibitor of GEMC1 and MCIDAS function [40][44].
During MCC differentiation, the biogenesis of hundreds of centrioles occurs through two different pathways: the canonical centriolar pathway, which utilizes pre-existing centrioles for new centriole generation, and the de novo deuterosome-mediated pathway [51][52]. Although a small fraction of centrioles is produced via the canonical centriolar pathway, the majority are thought to be amplified from deuterosomes. Deuterosomes are electron-dense, fibrogranular structures that contain several proteins required for centriole amplification, such as DEUP1 and coiled-coil domain-containing 78 (CCDC78) [48][53][54]. Although research on the origins and molecular components of deuterosomes is still ongoing, it has been reported that deuterosomes are nucleated from existing centrioles or spontaneously synthesized in the cytoplasm [55][56][57]. A recent study questioned the need for the deuterosome in MCC centriole amplification, since MCCs lacking deuterosomes are able to amplify the correct number of centrioles with normal kinetics [58].
Following release from the deuterosome or centrosomal centriole, the nascent centrioles migrate to the apical cell surface, dock at the plasma membrane, and mature into basal bodies to initiate cilium assembly. For efficient basal body docking to occur, small vesicles are recruited to the distal appendages of centrioles and then fuse to form a larger membranous cap called the ciliary vesicle [13][53][59]. Subsequently, the ciliary vesicle flattens and forms a bilayer membranous sheath around the developing ciliary axoneme that merges with the cell membrane, allowing the elongating cilium to protrude from the apical cell surface. Vesicular trafficking during ciliogenesis has been studied mainly using mammalian cultured cells with primary cilia. In early stages of ciliogenesis, the fusion of small vesicles into the ciliary vesicle is mediated by the EPS-15 homology domain-containing (EHD) family of membrane-shaping proteins, EHD1 and EHD3 [52][60]. The small GTPase Rab11 then recruits and activates Rabin8, a guanine nucleotide exchange factor (GEF) for Rab8, in the vicinity of the centrosome. Rabin8, in turn, promotes the recruitment and local activation of Rab8 to facilitate ciliary membrane growth and vesicular transport of ciliary proteins into the cilium [61][62]. Rab8 and Rabin8 also interact with the distal appendage protein CEP164 (also known as NPHP15), which acts as a molecular bridge between the mother centriole and components of the ciliary membrane assembly machinery [12]. Our group demonstrated that, in airway MCCs, the 15-kDa coiled-coil protein Chibby1 (Cby1) plays an important role in ciliary vesicle formation and basal body docking (Figure 2) [13][63]. Cby1−/− mice exhibit chronic upper airway infection, due to a markedly decreased number of multicilia and a complete absence of mucociliary clearance activity [63]. Cby1 and its interacting proteins localize to the base of cilia (Figure 2A). Cby1 is recruited to the distal appendages of basal bodies through its physical interaction with CEP164 and interacts with Rabin8 to facilitate the efficient assembly of ciliary vesicles (Figure 2B) [13][64]. Cby1 exists in a complex with the membrane remodeling proteins, Chibby1-interacting Bin/Amphiphysin/Rvs (BAR) domain-containing 1 and 2 (ciBAR1 and ciBAR2; previously known as FAM92A and FAM92B, respectively) [64][65]. Coexpression of ciBAR1 or ciBAR2 with Cby1 in mammalian cultured cells induces the formation of globular and tubular membrane structures, suggesting that the Cby1/ciBAR complex facilitates ciliogenesis through regulation of membrane-remodeling processes.
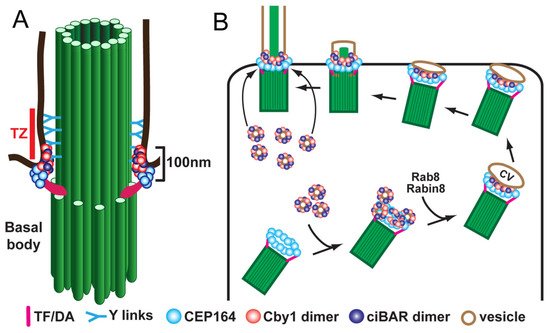
Figure 2. Model for localization (A) and function (B) of Cby1 and its associated proteins in airway MCCs. Cby1 clusters at the ciliary base as a ring with a diameter of 300 nm and a height of 100 nm [13]. Cby1 and its interactors are essential for the efficient docking of basal bodies to the apical membrane. TZ, transition zone; TF, transition fiber; DA, distal appendage; CV, ciliary vesicle.
The docking and planar polarization of basal bodies are controlled by the interplay between the planar cell polarity (PCP) pathway and the underlying actin and microtubule cytoskeleton [66][67][68][69][70][71]. The PCP pathway is a noncanonical Wnt pathway and has emerged as a critical regulator of MCC differentiation. In MCCs, the orientation of basal bodies is established via rotational polarity at the apical cell surface to enable synchronous beating of motile multicilia on each cell. PCP components localize to opposing membrane domains at the proximal and distal sides of cells [68][69]. The core PCP components include the evolutionarily conserved transmembrane proteins Frizzled, Van Gogh-like (Vangl, Van Gogh in Drosophila), Celsr (Flamingo in Drosophila) and cytoplasmic proteins Dishevelled, Prickle, and Inversin/Diversin (Diego in Drosophila). Mutations in the core PCP components lead to basal body docking defects and random orientation of basal bodies and ciliary beating in MCCs.
The extension and maintenance of cilia depend on the IFT machinery [18][19]. Along the cilium, anterograde IFT movement is mediated by kinesin-2 motors (from base to tip), whereas retrograde transport is powered by cytoplasmic dynein-2 (from tip to base). Fully developed cilia are highly dynamic and constantly undergo turnover with continuous transport of proteins and lipids in and out of cilia [72][73].
3. Male Reproductive Tract and Passage of Spermatozoa through Efferent Ducts
The male reproductive tract is composed of several highly convoluted tubular segments, each of which plays a crucial role in the development of fully functional spermatozoa (sperm) (Figure 3A). The testis, consisting of long convoluted seminiferous tubules, is the site of spermatogenesis [74][75][76]. From the testis, the spermatozoa are collected in the rete testis and are transported into the epididymis via the efferent ducts (EDs) [77][78]. The EDs contain specialized MCCs that prevent sperm from aggregating [79]. In the epididymis, sperm continue to mature and are finally stored in the cauda epididymis for eventual release via the vas deferens [80][81].
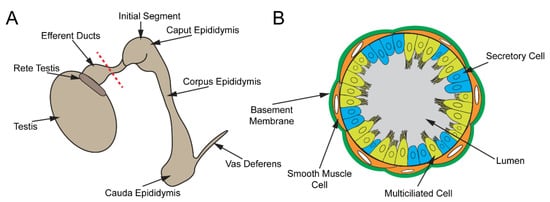
Figure 3. Male reproductive tract. (A) Cartoon schematic of the male reproductive tract. Sperm are produced in the seminiferous tubules of the testis, collected in the rete testis, and transported to the epididymis via EDs, where they mature. Mature sperm are stored in the cauda epididymis and released through the vas deferens. (B) Depiction of a cross-section of the ED along the red dashed line in (A). The ED epithelium consists of MCCs and secretory cells.
The development of spermatozoa takes place in the testis. The seminiferous tubules consist of multiple cell types, including spermatogenic cells, Sertoli cells, and peritubular myoid cells. The spermatogenic cycle occurs in waves, and different regions of the seminiferous tubules contain spermatogenic cells at varying steps of differentiation. Sertoli cells are nurse cells that aid spermatogenesis by anchoring developing spermatids to the seminiferous tubule epithelium and establishing the blood–testis barrier [82]. Peritubular myoid cells are smooth muscle-like cells that surround the seminiferous tubules and provide structural rigidity to the seminiferous tubules and contract to push spermatozoa and fluid into the rete testis.
Spermatogenesis is initiated by the asymmetric cell division of spermatogonial stem cells. Following several rounds of mitotic cell divisions, primary spermatocytes go through two rounds of meiosis to give rise to spermatids [75]. Spermatids then undergo spermiogenesis, in which round spermatids transform into elongated spermatozoa with a flagellum and a species-specific head shape [74]. Upon completion of spermiogenesis, the spermatozoa appear morphologically mature but lack motility and the capacity to fertilize an egg [83]. Sertoli cell secretions release spermatozoa into the lumen of the seminiferous tubules and coordinated contractions of peritubular myoid cells propel sperm into the rete testis [79].
Once collected in the rete testis, sperm transit into the EDs. The EDs originate from mesonephric tubules and are critical for concentrating the seminiferous fluid by reabsorbing water and ions and transporting sperm into the epididymis [78][84][85][86]. Three-dimensional reconstruction of mouse EDs revealed that EDs are 22.5 µm in mean radius and 81.9 mm in length [87]. Sperm transport through the EDs occurs within 45 min in rats [88]. The number of EDs in the mice varies from 2 to 5 [87][89][90][91]. In golden Syrian hamsters, there are six ducts [92]. In humans and other large mammals, EDs are more numerous and feed into the epididymis at various sites, whereas EDs in rodents collect into a singular duct (known as the common duct) prior to entering the epididymis [93].
This entry is adapted from the peer-reviewed paper 10.3390/cells11030341
References
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219.
- Goetz, S.C.; Anderson, K.V. The primary cilium: A signalling centre during vertebrate development. Nat. Rev. Genet. 2010, 11, 331–344.
- Hildebrandt, F.; Benzing, T.; Katsanis, N. Ciliopathies. N. Engl. J. Med. 2011, 364, 1533–1543.
- Hamada, H. Molecular and cellular basis of left–right asymmetry in vertebrates. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2020, 96, 273–296.
- Shinohara, K.; Hamada, H. Cilia in Left–Right Symmetry Breaking. Cold Spring Harb. Perspect. Biol. 2017, 9, a028282.
- Garcia, G.; Raleigh, D.R.; Reiter, J.F. How the Ciliary Membrane Is Organized Inside-Out to Communicate Outside-In. Curr. Biol. 2018, 28, R421–R434.
- Conduit, S.E.; Vanhaesebroeck, B. Phosphoinositide lipids in primary cilia biology. Biochem. J. 2020, 477, 3541–3565.
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26.
- Kumar, V.; Umair, Z.; Kumar, S.; Goutam, R.S.; Park, S.; Kim, J. The regulatory roles of motile cilia in CSF circulation and hydrocephalus. Fluids Barriers CNS 2021, 18, 31.
- Inaba, K.; Mizuno, K. Sperm dysfunction and ciliopathy. Reprod. Med. Biol. 2016, 15, 77–94.
- Sironen, A.; Shoemark, A.; Patel, M.; Loebinger, M.R.; Mitchison, H.M. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell Mol. Life Sci. 2020, 77, 2029–2048.
- Schmidt, K.N.; Kuhns, S.; Neuner, A.; Hub, B.; Zentgraf, H.; Pereira, G. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J. Cell Biol. 2012, 199, 1083–1101.
- Burke, M.C.; Li, F.-Q.; Cyge, B.; Arashiro, T.; Brechbuhl, H.M.; Chen, X.; Siller, S.S.; Weiss, M.A.; O’Connell, C.B.; Love, D.; et al. Chibby promotes ciliary vesicle formation and basal body docking during airway cell differentiation. J. Cell Biol. 2014, 207, 123–137.
- Wei, Q.; Ling, K.; Hu, J. The essential roles of transition fibers in the context of cilia. Curr. Opin. Cell Biol. 2015, 35, 98–105.
- Reiter, J.F.; Blacque, O.E.; Leroux, M.R. The base of the cilium: Roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012, 13, 608–618.
- Hsiao, Y.-C.; Tuz, K.; Ferland, R.J. Trafficking in and to the primary cilium. Cilia 2012, 1, 4.
- Emmer, B.T.; Maric, D.; Engman, D.M. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J. Cell Sci. 2010, 123, 529–536.
- Rosenbaum, J.L.; Witman, G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002, 3, 813–825.
- Ishikawa, H.; Marshall, W.F. Intraflagellar Transport and Ciliary Dynamics. Cold Spring Harb. Perspect. Biol. 2017, 9, a021998.
- Vladar, E.K.; Stearns, T. Molecular characterization of centriole assembly in ciliated epithelial cells. J. Cell Biol. 2007, 178, 31–42.
- Dawe, H.R.; Farr, H.; Gull, K. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J. Cell Sci. 2007, 120, 7–15.
- Nigg, E.A.; Raff, J.W. Centrioles, centrosomes, and cilia in health and disease. Cell 2009, 139, 663–678.
- Pazour, G.J.; Quarmby, L.; Smith, A.O.; Desai, P.B.; Schmidts, M. Cilia in cystic kidney and other diseases. Cell Signal. 2020, 69, 109519.
- Ma, M.; Gallagher, A.-R.; Somlo, S. Ciliary Mechanisms of Cyst Formation in Polycystic Kidney Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a028209.
- Leigh, M.W.; Pittman, J.E.; Carson, J.L.; Ferkol, T.W.; Dell, S.D.; Davis, S.D.; Knowles, M.R.; Zariwala, M.A. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet. Med. 2009, 11, 473–487.
- Horani, A.; Ferkol, T.W.; Dutcher, S.K.; Brody, S.L. Genetics and biology of primary ciliary dyskinesia. Paediatr. Respir. Rev. 2016, 18, 18–24.
- Legendre, M.; Zaragosi, L.-E.; Mitchison, H.M. Motile cilia and airway disease. Semin. Cell Dev. Biol. 2021, 110, 19–33.
- Lee, L.; Ostrowski, L.E. Motile cilia genetics and cell biology: Big results from little mice. Cell Mol. Life Sci. 2021, 78, 769–797.
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile ciliopathies. Nat. Rev. Dis. Primers 2020, 6, 77.
- Cibois, M.; Luxardi, G.; Chevalier, B.; Thomé, V.; Mercey, O.; Zaragosi, L.-E.; Barbry, P.; Pasini, A.; Marcet, B.; Kodjabachian, L. BMP signalling controls the construction of vertebrate mucociliary epithelia. Development 2015, 142, 2352–2363.
- Guseh, J.S.; Bores, S.A.; Stanger, B.Z.; Zhou, Q.; Anderson, W.J.; Melton, D.A.; Rajagopal, J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 2009, 136, 1751–1759.
- Tsao, P.-N.; Vasconcelos, M.; Izvolsky, K.I.; Qian, J.; Lu, J.; Cardoso, W.V. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 2009, 136, 2297–2307.
- Nishimura, Y.; Hamazaki, T.S.; Komazaki, S.; Kamimura, S.; Okochi, H.; Asashima, M. Ciliated cells differentiated from mouse embryonic stem cells. Stem Cells 2006, 24, 1381–1388.
- Chevalier, B.; Adamiok, A.; Mercey, O.; Revinski, D.R.; Zaragosi, L.-E.; Pasini, A.; Kodjabachian, L.; Barbry, P.; Marcet, B. miR-34/449 control apical actin network formation during multiciliogenesis through small GTPase pathways. Nat. Commun. 2015, 6, 8386.
- Wu, J.; Bao, J.; Kim, M.; Yuan, S.; Tang, C.; Zheng, H.; Mastick, G.S.; Xu, C.; Yan, W. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E2851–E2857.
- Marcet, B.; Chevalier, B.; Coraux, C.; Kodjabachian, L.; Barbry, P. MicroRNA-based silencing of Delta/Notch signaling promotes multiple cilia formation. Cell Cycle 2011, 10, 2858–2864.
- Marcet, B.; Chevalier, B.; Luxardi, G.; Coraux, C.; Zaragosi, L.-E.; Cibois, M.; Robbe-Sermesant, K.; Jolly, T.; Cardinaud, B.; Moreilhon, C.; et al. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat. Cell Biol. 2011, 13, 693–699.
- Ma, L.; Quigley, I.; Omran, H.; Kintner, C. Multicilin drives centriole biogenesis via E2f proteins. Genes Dev. 2014, 28, 1461–1471.
- Stubbs, J.L.; Vladar, E.K.; Axelrod, J.D.; Kintner, C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat. Cell Biol. 2012, 14, 140–147.
- Arbi, M.; Pefani, D.E.; Kyrousi, C.; Lalioti, M.E.; Kalogeropoulou, A.; Papanastasiou, A.D.; Taraviras, S.; Lygerou, Z. GemC1 controls multiciliogenesis in the airway epithelium. EMBO Rep. 2016, 17, 400–413.
- Arbi, M.; Pefani, D.-E.; Taraviras, S.; Lygerou, Z. Controlling centriole numbers: Geminin family members as master regulators of centriole amplification and multiciliogenesis. Chromosoma 2018, 127, 151–174.
- Zhou, F.; Narasimhan, V.; Shboul, M.; Chong, Y.L.; Reversade, B.; Roy, S. Gmnc is a master regulator of the multiciliated cell differentiation program. Curr. Biol. 2015, 25, 3267–3273.
- Kyrousi, C.; Arbi, M.; Pilz, G.A.; Pefani, D.E.; Lalioti, M.E.; Ninkovic, J.; Gotz, M.; Lygerou, Z.; Taraviras, S. Mcidas and GemC1 are key regulators for the generation of multiciliated ependymal cells in the adult neurogenic niche. Development 2015, 142, 3661–3674.
- Terré, B.; Piergiovanni, G.; Segura-Bayona, S.; Gil-Gómez, G.; Youssef, S.A.; Attolini, C.S.-O.; Wilsch-Bräuninger, M.; Jung, C.; Rojas, A.; Marjanović, M.; et al. GEMC 1 is a critical regulator of multiciliated cell differentiation. EMBO J. 2016, 35, 942–960.
- Lu, H.; Anujan, P.; Zhou, F.; Zhang, Y.; Chong, Y.L.; Bingle, C.D.; Roy, S. Mcidas mutant mice reveal a two-step process for the specification and differentiation of multiciliated cells in mammals. Development 2019, 146, dev172643.
- Vladar, E.K.; Mitchell, B.J. It’s a family act: The geminin triplets take center stage in motile ciliogenesis. EMBO J. 2016, 35, 904–906.
- Kim, S.; Ma, L.; Shokhirev, M.N.; Quigley, I.; Kintner, C. Multicilin and activated E2f4 induce multiciliated cell differentiation in primary fibroblasts. Sci. Rep. 2018, 8, 12369.
- Zhao, H.; Zhu, L.; Zhu, Y.; Cao, J.; Li, S.; Huang, Q.; Xu, T.; Huang, X.; Yan, X.; Zhu, X. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat. Cell Biol. 2013, 15, 1434–1444.
- Marshall, C.B.; Mays, D.J.; Beeler, J.S.; Rosenbluth, J.M.; Boyd, K.L.; Guasch, G.L.S.; Shaver, T.M.; Tang, L.J.; Liu, Q.; Shyr, Y.; et al. P73 Is Required for Multiciliogenesis and Regulates the Foxj1-Associated Gene Network. Cell Rep. 2016, 14, 2289–2300.
- Nemajerova, A.; Kramer, D.; Siller, S.S.; Herr, C.; Shomroni, O.; Pena, T.; Suazo, C.G.; Glaser, K.; Wildung, M.; Steffen, H.; et al. TAp73 is a central transcriptional regulator of airway multiciliogenesis. Genes Dev. 2016, 30, 1300–1312.
- Spassky, N.; Meunier, A. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 2017, 18, 423–436.
- Breslow, D.K.; Holland, A.J. Mechanism and Regulation of Centriole and Cilium Biogenesis. Annu. Rev. Biochem. 2019, 88, 691–724.
- Sorokin, S.P. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 1968, 3, 207–230.
- Dehring, D.A.K.; Vladar, E.K.; Werner, M.E.; Mitchell, J.W.; Hwang, P.; Mitchell, B.J. Deuterosome-mediated centriole biogenesis. Dev. Cell 2013, 27, 103–112.
- Al Jord, A.; Lemaître, A.-I.; Delgehyr, N.; Faucourt, M.; Spassky, N.; Meunier, A. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature 2014, 516, 104–107.
- Zhao, H.; Chen, Q.; Fang, C.; Huang, Q.; Zhou, J.; Yan, X.; Zhu, X. Parental centrioles are dispensable for deuterosome formation and function during basal body amplification. EMBO Rep. 2019, 20, e46735.
- Nanjundappa, R.; Kong, D.; Shim, K.; Stearns, T.; Brody, S.L.; Loncarek, J.; Mahjoub, M.R. Regulation of cilia abundance in multiciliated cells. eLife 2019, 8, e44039.
- Mercey, O.; Levine, M.S.; LoMastro, G.M.; Rostaing, P.; Brotslaw, E.; Gomez, V.; Kumar, A.; Spassky, N.; Mitchell, B.J.; Meunier, A.; et al. Massive centriole production can occur in the absence of deuterosomes in multiciliated cells. Nat. Cell Biol. 2019, 21, 1544–1552.
- Li, F.-Q.; Siller, S.S.; Takemaru, K.-I. Basal body docking in airway ciliated cells. Oncotarget 2015, 6, 19944–19945.
- Lü, Q.; Insinna, C.; Ott, C.; Stauffer, J.; Pintado, P.A.R.; Rahajeng, J.; Baxa, U.; Walia, V.; Cuenca, A.; Hwang, Y.-S.; et al. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat. Cell Biol. 2015, 17, 228–240.
- Feng, S.; Knödler, A.; Ren, J.; Zhang, J.; Zhang, X.; Hong, Y.; Huang, S.; Peränen, J.; Guo, W. A Rab8 Guanine Nucleotide Exchange Factor-Effector Interaction Network Regulates Primary Ciliogenesis. J. Biol. Chem. 2012, 287, 15602–15609.
- Westlake, C.J.; Baye, L.M.; Nachury, M.V.; Wright, K.J.; Ervin, K.E.; Phu, L.; Chalouni, C.; Beck, J.S.; Kirkpatrick, D.S.; Slusarski, D.C.; et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc. Natl. Acad. Sci. USA 2011, 108, 2759–2764.
- Voronina, V.A.; Takemaru, K.-I.; Treuting, P.; Love, D.; Grubb, B.R.; Hajjar, A.M.; Adams, A.; Li, F.-Q.; Moon, R.T. Inactivation of Chibby affects function of motile airway cilia. J. Cell Biol. 2009, 185, 225–233.
- Siller, S.S.; Sharma, H.; Li, S.; Yang, J.; Zhang, Y.; Holtzman, M.J.; Winuthayanon, W.; Colognato, H.; Holdener, B.C.; Li, F.-Q.; et al. Conditional knockout mice for the distal appendage protein CEP164 reveal its essential roles in airway multiciliated cell differentiation. PLoS Genet. 2017, 13, e1007128.
- Li, F.-Q.; Chen, X.; Fisher, C.; Siller, S.S.; Zelikman, K.; Kuriyama, R.; Takemaru, K.-I. BAR Domain-Containing FAM92 Proteins Interact with Chibby1 To Facilitate Ciliogenesis. Mol. Cell. Biol. 2016, 36, 2668–2680.
- Meunier, A.; Azimzadeh, J. Multiciliated Cells in Animals. Cold Spring Harb. Perspect. Biol. 2016, 8, a028233.
- Werner, M.E.; Hwang, P.; Huisman, F.; Taborek, P.; Yu, C.C.; Mitchell, B.J. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J. Cell Biol. 2011, 195, 19–26.
- Vladar, E.K.; Königshoff, M. Noncanonical Wnt planar cell polarity signaling in lung development and disease. Biochem. Soc. Trans. 2020, 48, 231–243.
- Ohata, S.; Alvarez-Buylla, A. Planar Organization of Multiciliated Ependymal (E1) Cells in the Brain Ventricular Epithelium. Trends Neurosci. 2016, 39, 543–551.
- Gegg, M.; Böttcher, A.; Burtscher, I.; Hasenoeder, S.; Van Campenhout, C.; Aichler, M.; Walch, A.; Grant, S.G.; Lickert, H. Flattop regulates basal body docking and positioning in mono- and multiciliated cells. eLife 2014, 3, e03842.
- Park, T.J.; Mitchell, B.J.; Abitua, P.B.; Kintner, C.; Wallingford, J.B. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 2008, 40, 871–879.
- Lechtreck, K.F.; Van De Weghe, J.C.; Harris, J.A.; Liu, P. Protein transport in growing and steady-state cilia. Traffic 2017, 18, 277–286.
- Werner, S.; Pimenta-Marques, A.; Bettencourt-Dias, M. Maintaining centrosomes and cilia. J. Cell Sci. 2017, 130, 3789–3800.
- De Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13, 1–8.
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17.
- Russell, L.D.; Ettlin, R.A.; Hikim, A.P.S.; Clegg, E.D. Histological and Histopathological Evaluation of the Testis. Int. J. Androl. 1993, 16, 83.
- Hess, R.A. Small tubules, surprising discoveries: From efferent ductules in the turkey to the discovery that estrogen receptor alpha is essential for fertility in the male. Anim. Reprod. 2015, 12, 7–23.
- Hess, R.A. Efferent Ductules: Structure and Function. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Oxford, UK, 2018; pp. 270–278.
- Yuan, S.; Liu, Y.; Peng, H.; Tang, C.; Hennig, G.W.; Wang, Z.; Wang, L.; Yu, T.; Klukovich, R.; Zhang, Y.; et al. Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence. Proc. Natl. Acad. Sci. USA 2019, 116, 3584–3593.
- Bork, K.; Chevrier, C.; Paquignon, M.; Jouannet, P.; Dacheux, J. Flagellar motility and movement of boar spermatozoa during epididymal transit. Reprod. Nutr. Dev. 1988, 28, 1307–1315.
- Dacheux, J.-L.; Dacheux, F. New insights into epididymal function in relation to sperm maturation. Reproduction 2014, 147, R27–R42.
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416.
- Lacham-Kaplan, O.; Trounson, A. The effects of the sperm motility activators 2-deoxyadenosine and pentoxifylline used for sperm micro-injection on mouse and human embryo development. Hum. Reprod. 1993, 8, 945–952.
- Clulow, J.; Jones, R.; Hansen, L.; Man, S. Fluid and electrolyte reabsorption in the ductuli efferentes testis. J. Reprod. Fertil. Suppl. 1998, 53, 1–14.
- Clulow, J.; Jones, R.C.; Hansen, L.A. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: Comparisons with the homologous metanephric proximal tubule. Exp. Physiol. 1994, 79, 915–928.
- Veeramachaneni, D.N.; Amann, R.P.; Palmer, J.S.; Hinton, B.T. Proteins in luminal fluid of the ram excurrent ducts: Changes in composition and evidence for differential endocytosis. J. Androl. 1990, 11, 140–154.
- Lambot, M.-A.H.; Mendive, F.; Laurent, P.; Van Schoore, G.; Noël, J.-C.; Vanderhaeghen, P.; Vassart, G. Three-dimensional reconstruction of efferent ducts in wild-type and Lgr4 knock-out mice. Anat. Rec. 2009, 292, 595–603.
- English, H.F.; Dym, M. The time required for materials injected into the rete testis to reach points in the caput epididymis of the rat and observations on the absorption of cationic ferritin. Ann. N. Y. Acad. Sci. 1982, 383, 445–446.
- Benoit, J. Recherches anatomiques, cytologiques et histophysiologiques, sur les voies excrétices du testicules chez les mammiferes. Archs. Anat. Histol. Embryol. 1926, 5, 173–412.
- Cunningham, J. On ligature of the vas deferens in the cat and researches on the efferent ducts of the testis in cat, rat and mouse. J. Exp. Biol. 1928, 6, 12–25.
- Barack, B.M. Transport of spermatozoa from seminiferous tubules to epididymis in the mouse: A histological and quantitative study. J. Reprod. Fertil. 1968, 16, 35–48.
- Ford, J., Jr.; Carnes, K.; Hess, R.A. Ductuli efferentes of the male Golden Syrian hamster reproductive tract. Andrology 2014, 2, 510–520.
- Hemeida, N.A.; Sack, W.O.; McEntee, K. Ductuli efferentes in the epididymis of boar, goat, ram, bull, and stallion. Am. J. Vet. Res. 1978, 39, 1892–1900.
This entry is offline, you can click here to edit this entry!
