Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
The proteasome is responsible for selective degradation of most cellular proteins. Abundantly present in the cell, proteasomes not only diffuse in the cytoplasm and the nucleus but also associate with the chromatin, cytoskeleton, various membranes and membraneless organelles/condensates. How and why the proteasome gets to these specific subcellular compartments remains poorly understood, although increasing evidence supports the hypothesis that intracellular localization may have profound impacts on the activity, substrate accessibility and stability/integrity of the proteasome.
- proteasome
- membrane
- nucleus
- condensate
- ubiquitination
- myristoylation
1. Introduction
The 26S proteasome is situated at the core of the ubiquitin–proteasome system (UPS), responsible for selective degradation of the majority of cellular proteins in eukaryotes. For over three decades since its discovery, the proteasome has been thoroughly studied with regard to its composition, structure, activity, regulation and relation to health and disease. The fully assembled 26S proteasome holoenzyme consists of a 20S core particle (CP, formed by homologous α and β type subunits) and one or two 19S regulatory particles (RP, formed by six ATPase subunits called Rpt1-6, and thirteen non-ATPase subunits known as Rpns) [1][2][3][4][5][6][7][8][9][10]. Recent structural studies have significantly furthered our knowledge about how the proteasome recognizes and processes ubiquitinated substrates [6][11][12]. The success of proteasome inhibitors (e.g., Bortezomib/Velcade®) in treating multiple myeloma [13] has spurred intensive research on developing proteasome-targeting compounds for therapeutic uses toward cancer and autoimmune diseases, whereas (re-)activating the proteasome by small molecules has also emerged as an attractive strategy for alleviating symptoms associated with neurodegeneration and aging [14][15][16][17][18][19]. A better understanding of the function and regulation of the proteasome is of great biological and clinical importance.
As a soluble and highly abundant macromolecular complex [20][21][22], the proteasome resides in both the nucleus and cytoplasm of a cell and has been found associated with various subcellular structures, including the chromatin, cytoskeleton, nuclear envelope, plasma membrane, the cytosolic side of membrane-bound organelles and membraneless organelles/condensates (see below). Despite their pervasive presence, proteasomes are not evenly distributed in all cells. On a global scale, asymmetric cell division can lead to unequal inheritance of proteasomes between the daughter cells [23][24][25][26]. The specific subcellular localizations of proteasomes are often cell type- and growth status-dependent and dynamically regulated under both basal and stimulated/stress conditions [27][28][29]. A classic example is that in yeast, proteasomes are predominantly present in the nucleus of proliferating cells; but upon quiescence or carbon starvation, nuclear proteasomes are rapidly exported to the cytosol, where they are concentrated in a membraneless structure called proteasome storage granule (PSG) [30]. PSGs quickly resolve when yeast cells resume growth in nutrient-rich media and proteasomes re-gather in the nucleus. This reversible process is believed to protect the proteasome repertoire from autophagic degradation under stress conditions, while allowing them to regain function as soon as the stress is relieved [30][31].
Proteasomes also exist extracellularly. Original studies have shown that secreted proteasomes from ascidian sperms can digest vitelline coat proteins outside the egg and are required for egg penetration and fertilization [32][33][34]. Circulating proteasomes (c-proteasomes) were also found in humans around the same time [35], which has been confirmed by a series of subsequent studies (see reviews [36][37][38] and references therein). Present in the blood as well as other bodily fluids, these c-proteasomes are mostly in the form of 20S, probably due to the low-ATP extracellular environment that does not support RP–CP association [39][40]. Nonetheless, they are enzymatically active, and elevation of their levels is often correlated with either malignancy or tissue injury/damage, making them a promising biomarker for disease diagnosis [36][37][38]. How the proteasomes exit the cell remains a matter of debate, although a likely mechanism is via exosome-mediated non-conventional secretion [36][41]. The pathophysiological roles and regulatory mechanisms of extracellular proteasomes have yet to be fully understood.
Various mechanisms have been identified to target protein substrates to different subcellular regions for proteasomal degradation [42][43][44][45][46][47][48][49][50]. On the flip side, proteasomes should be available at the site of degradation or can be mobilized to meet the substrates. In addition to the examples introduced above, the dynamic localizations of the proteasome have been extensively studied (particularly in yeast) and summarized in a series of reviews [51][52][53][54][55]. Here, I will focus on the latest findings about nuclear-localized and membrane-associated proteasomes in mammalian cells and discuss the targeting mechanisms, biological functions, as well as regulations of proteasomes at these specific compartments.
2. Proteasomes at the Membranes
Membrane localization of the proteasome has been documented since the early 1990′s [56][57]. Numerous subsequent studies have documented proteasomes in close contact with nuclear envelope-ER [58][59][60][61][62][63][64][65], the Golgi apparatus [66][67], endosomes [68][69], plasma membrane [70][71], mitochondria [64][72][73][74][75][76][77] and so on. Proteasomes at the membranes are particularly important for organelle quality control processes, such as ER-associated degradation (ERAD), endosome and Golgi-associated degradation (EGAD) and mitophagy [61][66][67][78][79][80][81][82]. In addition, proteasomes located at neuronal synapses are also critical for neurotransmission and synaptic plasticity [83][84][85][86][87]. In these cases, the proteasome associates peripherally with the membrane by binding to membrane-resident proteins. This can occur directly between proteasome subunits and membrane proteins. Alternatively, proteasomes can be indirectly recruited to the membrane through binding to the ubiquitin moiety of modified membrane proteins, proteasome-interacting proteins (sometimes in concert with motor proteins and cytoskeleton) [88][89] or even RNAs that function as protein scaffolds [90]. Together, these represent the most common mode of proteasome–membrane interaction, while the proteasome can also locate to the membrane in two other ways, as elaborated below (Figure 1).
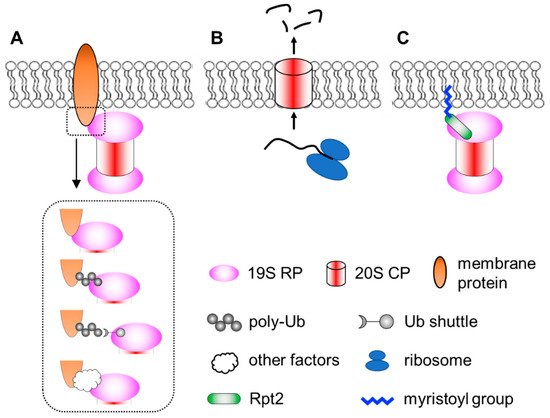
Figure 1. A simplified view of different modes of proteasome–membrane interaction. (A) In most cases, proteasomes attach to the membrane by directly or indirectly binding to resident membrane proteins, which may be modified by ubiquitination. (B) Neuronal membrane proteasomes (NMP) can degrade nascent, unfolded polypeptides. (C) Proteasomes tethered to the membrane via N-myristoylated Rpt2, which is evolutionarily conserved from yeast to human.
2.1. Neuronal Membrane Proteasomes
Ramachandran et al. reported a surprising type of membrane-associated proteasomes designated as the neuronal membrane proteasomes (NMPs) [91][92]. As the name suggests, these proteasomes are found at pre- and post-synaptic plasma membranes in neurons, which were confirmed by immunogold electron microscopy (IEM), surface biotinylation, immunofluorescence imaging with antibody feeding and proteinase protection assays. NMPs are thought to be comprised of the 20S CP only, since no 19S components (such as Rpt5 or Rpn1) were found by IEM in these particular membrane proteasomes. NMPs are capable of degrading newly synthesized polypeptides, which are still unfolded, to short peptides. More fascinatingly, the authors showed that these peptide products could exit the cells through NMPs and be released into the synaptic cleft to function as neurotransmitters. Therefore, NMPs function not only as a protein degrader but also a new form of membrane channel to mediate cell–cell communications [93]. Although these findings were very unique and intriguing, the molecular and biochemical details of the NMPs remain unclear. First, it is curious that the 20S CP, which is soluble and hydrophilic, could be fully embedded within the hydrophobic membrane. How is the CP targeted to the plasma membrane and how does it overcome the energy barrier to traverse the lipid bilayer? It was proposed that glycoproteins, such as GPM6, could facilitate this process [92], but a clear mechanistic explanation is still needed. Second, does the NMP exhibit any substrate selectivity? The proposed role of NMPs in cleaving nascent proteins suggests that substrate availability depends on localized protein synthesis by ribosomes in the vicinity [91]. However, if the NMP complex also contained auxiliary factors yet to be identified, it might recognize and process folded protein substrates as well. On the other hand, the recent discovery that the 20S CP can by itself degrade ubiquitinated proteins [94] also implies that NMPs may have a broader range of substrates. A following question is the molecular composition and regulatory mechanisms of the NMPs. Finally, what is the function of NMP in vivo? Additionally, how can we specifically maneuver it for research and therapeutic purposes without affecting the bulk of proteasomes inside the cell? Answering these questions will depend on new technical advances in imaging, chemical biology, proteomics, structural biology and genetic models, which makes it challenging but also rewarding at the same time.
2.2. Membrane Targeting of Proteasomes by N-Myristoylation
A third means of targeting the proteasome to the membrane is through lipid modification. N-myristoylation of the Rpt2 subunit has been observed by mass spectrometry in multiple species, ranging from yeast to plants to mammals [95][96][97][98][99][100][101][102]. Typically, N-myristoylation occurs co-translationally on nascent polypeptides still bound to the ribosome, where the 14-carbon saturated fatty acyl group is covalently linked to the second amino acid (almost always a Gly) after the initiator methionine is removed by methionyl aminopeptidase [103][104][105]. Notably, among all proteasome subunits of mammalian cells, Rpt2 is the only one that begins with Met-Gly, serving as the only site of the entire proteasome complex for N-myristoylation. This MG sequence of Rpt2 is strictly conserved from yeast to human, suggesting that Rpt2 is likely to be myristoylated in all species. In yeast, myristoylated Rpt2 has been shown to target proteasomes to the nuclear envelope, which is required for nuclear protein quality control [97][98]. Blocking this modification with the Rpt2-ΔG or Rpt2-G2A mutations causes mislocalization of nuclear proteasomes to the cytosol.
The role of Rpt2 myristoylation in higher organisms has not been rigorously investigated, despite Rpt2 being one of the most abundantly myristoylated proteins in human cells [102]. Our recent work demonstrated that wild-type human Rpt2 proficient for myristoylation was found at the plasma membrane, with some distribution at membrane-bound organelles as well. Membrane localization was abolished by the same ΔG/G2A mutations of human Rpt2. However, in stark contrast with results from yeast, loss of Rpt2 myristoylation in mammalian cells led to Rpt2 enrichment in the nucleus [106]. A serendipitous finding was that myristoylation-mediated membrane association is a prerequisite for Rpt2 phosphorylation at Tyr439 (Y439) by the tyrosine kinase Src, which itself is a well-established myristoylated protein tethered to the membrane [106][107]. Moreover, Rpt2-Y439 phosphorylation could be reversed by the phosphotyrosine phosphatase PTPN2 (also known as T cell PTP or TC-PTP). PTPN2 has multiple splicing isoforms. Rpt2-pY439 could only be dephosphorylated by the membrane-bound isoform of PTPN2 known as TC48, but not by the nuclear isoform TC45 [106]. Hence, the kinase, phosphatase and substrate are all placed in the same neighborhood confined by the membrane.
The biochemical consequence of Rpt2-Y439 phosphorylation is readily conceivable, as it is the very tyrosine residue within the highly conserved HbYX tail (hydrophobic residue—Tyr—any amino acid) of Rpt2 required for RP–CP association. Rpt2-Y439 is the most frequently detected pTyr site of all 19S subunits. The phosphorylation was seen in the developing rat brain but more evidently detected in cancer cells with hyperactive Src [106]. Src-mediated Rpt2-Y439 phosphorylation selectively inhibited the activity of membrane-associated proteasomes as demonstrated by a membrane-targeted reporter protein, MyrRpt2-GFPodc. On the contrary, the Src-specific inhibitor saracatinib/AZD0530 blocked Y439 phosphorylation and enhanced proteasomal degradation of membrane-bound substrates. Importantly, this seemed to be an integral part of the anti-cancer effects of saracatinib, since cancer cells expressing the nonphosphorylatable Y439F mutant were more resistant to this drug, both in vitro and in vivo [106]. Thus, reversible phosphorylation of Rpt2-Y439 provides a unique example of localized regulation of membrane-associated proteasomes.
3. Current Insights
Proteasome localization is highly dynamic within the cell and may be remarkably heterogeneous between cell types. This is an important basis of compartmentalized protein degradation that is widely conserved through evolution. Nonetheless, we have seen differences between yeast and mammalian cells where the behavior and fate of the proteasome are differentially controlled by specific factors. We are just beginning to get in-depth understanding of intracellular proteasome targeting and trafficking in higher organisms, and it remains a daunting mission to obtain a complete picture of localized function and regulation of the proteasome across different cell types, species and growth/stress conditions. A yet more challenging task would be to confirm these findings in vivo and to develop new tools for “site-specific” manipulation of proteasomes at any particular location in a cell.
A prerequisite for achieving these goals is a deeper and better characterization of proteasome composition, modification, interactome and its microenvironment within a cell. Researchers have been empowered by state-of-the-art techniques, including proximity labeling (e.g., BioID/TurboID, APEX, PUP-IT) [108][109][110], quantitative proteomics, super-resolution imaging and cryo-electron tomography (cryo-ET) [21][22][111][61][63] to probe and catalog the contents of proteasome-containing subcellular structures. Chemical biology approaches involving metabolic labeling, click chemistry, genetic code expansion and cross-linking mass spectrometry (XL-MS) have provided critical insights into proteasome modification and assembly [112][102][113][114][115]. Commonly used reporter proteins (e.g., GFPu, GFPodc, UbG76V-GFP, UBL-CP8-35) can be engineered to reflect local proteasome activity at defined compartments [106][116][117][118], while knock-in mice bearing fluorescence protein-tagged proteasome subunits would be valuable to monitor proteasome distribution and dynamics in vivo [119]. With classic yeast genetics and CRISPR screens, many more regulators of the proteasome are expected to be uncovered [120][121].
Finally, some further possibilities may be speculated. 1. In addition to the above discussed, what other mechanisms may be used for proteasome targeting? Can we alter proteasome localization (and function) pharmacologically, optically, mechanically, magnetically or acoustically [122]? Does lipid modification (i.e., N-myristoylation) promote exosomal secretion of the proteasome, as has been shown with palmitoylated ACE2 [123]? Can we design “Proteasome-TACs” that recruit proteasomes directly to the substrates (or vice versa) for therapeutic use? A recently discovered small circular RNA seemed to do precisely that [90]. Along this line, since proteasome subunits have been identified as RNA-binding proteins [124][125], can RNAs act as molecular tethers between the proteasome and chromatin or other proteins [126]? 2. As mentioned earlier, cancer cells show reduced SIPAN formation [127], and tyrosine phosphorylation of membrane-bound Rpt2 is relevant to the anti-cancer effect of saracatinib [106]. This makes one wonder whether proteasome (mis)localization can be considered as a biomarker for disease diagnosis and treatment. Moreover, it is unclear whether subcellular localization of the proteasome is altered in multiple myeloma patients after proteasome inhibitor treatment, or in patients with proteasome-associated autoinflammatory syndrome (PRAAS) who carry congenital mutations in proteasome genes [128][129]. Relocation of the proteasome may cause changes to the local proteome and rewire intracellular signaling, which may lead to a different kind of proteasome-oriented therapy.
This entry is adapted from the peer-reviewed paper 10.3390/biom12020229
References
- Collins, G.A.; Goldberg, A.L. The Logic of the 26S Proteasome. Cell 2017, 169, 792–806.
- Bard, J.A.M.; Goodall, E.A.; Greene, E.R.; Jonsson, E.; Dong, K.C.; Martin, A. Structure and function of the 26S proteasome. Annu. Rev. Biochem. 2018, 87, 697–724.
- Budenholzer, L.; Cheng, C.L.; Li, Y.; Hochstrasser, M. Proteasome structure and assembly. J. Mol. Biol. 2017, 429, 3500–3524.
- Murata, S.; Yashiroda, H.; Tanaka, K. Molecular mechanisms of proteasome assembly. Nat. Rev. Mol. Cell Biol. 2009, 10, 104–115.
- Finley, D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 2009, 78, 477–513.
- Mao, Y. Structure, dynamics and function of the 26S proteasome. Subcell Biochem. 2021, 96, 1–151.
- Finley, D.; Prado, M.A. The proteasome and its network: Engineering for adaptability. Cold Spring Harb. Perspect. Biol. 2020, 12, a033985.
- Schmidt, M.; Finley, D. Regulation of proteasome activity in health and disease. Biochim. Biophys. Acta 2014, 1843, 13–25.
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479.
- Marshall, R.S.; Vierstra, R.D. Dynamic regulation of the 26S proteasome: From synthesis to degradation. Front. Mol. Biosci. 2019, 6, 40.
- de la Pena, A.H.; Goodall, E.A.; Gates, S.N.; Lander, G.C.; Martin, A. Substrate-engaged 26S proteasome structures reveal mechanisms for ATP-hydrolysis-driven translocation. Science 2018, 362, eaav0725.
- Dong, Y.; Zhang, S.; Wu, Z.; Li, X.; Wang, W.L.; Zhu, Y.; Stoilova-McPhie, S.; Lu, Y.; Finley, D.; Mao, Y. Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome. Nature 2019, 565, 49–55.
- Goldberg, A.L. Development of proteasome inhibitors as research tools and cancer drugs. J. Cell Biol. 2012, 199, 583–588.
- Kisselev, A.F. Site-specific proteasome inhibitors. Biomolecules 2022, 12, 54.
- Tundo, G.R.; Sbardella, D.; Santoro, A.M.; Coletta, A.; Oddone, F.; Grasso, G.; Milardi, D.; Lacal, P.M.; Marini, S.; Purrello, R.; et al. The proteasome as a druggable target with multiple therapeutic potentialities: Cutting and non-cutting edges. Pharmacol. Ther. 2020, 213, 107579.
- Leestemaker, Y.; de Jong, A.; Witting, K.F.; Penning, R.; Schuurman, K.; Rodenko, B.; Zaal, E.A.; van de Kooij, B.; Laufer, S.; Heck, A.J.R.; et al. Proteasome activation by small molecules. Cell Chem. Biol. 2017, 24, 725–736.e7.
- Goldberg, A.L.; Kim, H.T.; Lee, D.; Collins, G.A. Mechanisms that activate 26S proteasomes and enhance protein degradation. Biomolecules 2021, 11, 779.
- Njomen, E.; Tepe, J.J. Proteasome activation as a new therapeutic approach to target proteotoxic disorders. J. Med. Chem. 2019, 62, 6469–6481.
- He, Y.; Guo, X.; Yu, Z.H.; Wu, L.; Gunawan, A.M.; Zhang, Y.; Dixon, J.E.; Zhang, Z.Y. A potent and selective inhibitor for the UBLCP1 proteasome phosphatase. Bioorg. Med. Chem. 2015, 23, 2798–2809.
- Besche, H.C.; Goldberg, A.L. Affinity purification of mammalian 26S proteasomes using an ubiquitin-like domain. Methods Mol. Biol. 2012, 832, 423–432.
- Pack, C.G.; Yukii, H.; Toh-e, A.; Kudo, T.; Tsuchiya, H.; Kaiho, A.; Sakata, E.; Murata, S.; Yokosawa, H.; Sako, Y.; et al. Quantitative live-cell imaging reveals spatio-temporal dynamics and cytoplasmic assembly of the 26S proteasome. Nat. Commun. 2014, 5, 3396.
- Asano, S.; Fukuda, Y.; Beck, F.; Aufderheide, A.; Forster, F.; Danev, R.; Baumeister, W. Proteasomes. A molecular census of 26S proteasomes in intact neurons. Science 2015, 347, 439–442.
- Chang, J.T.; Ciocca, M.L.; Kinjyo, I.; Palanivel, V.R.; McClurkin, C.E.; Dejong, C.S.; Mooney, E.C.; Kim, J.S.; Steinel, N.C.; Oliaro, J.; et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity 2011, 34, 492–504.
- Chang, J.T.; Palanivel, V.R.; Kinjyo, I.; Schambach, F.; Intlekofer, A.M.; Banerjee, A.; Longworth, S.A.; Vinup, K.E.; Mrass, P.; Oliaro, J.; et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 2007, 315, 1687–1691.
- Moore, D.L.; Pilz, G.A.; Araúzo-Bravo, M.J.; Barral, Y.; Jessberger, S. A mechanism for the segregation of age in mammalian neural stem cells. Science 2015, 349, 1334–1338.
- Ogrodnik, M.; Salmonowicz, H.; Brown, R.; Turkowska, J.; Średniawa, W.; Pattabiraman, S.; Amen, T.; Abraham, A.C.; Eichler, N.; Lyakhovetsky, R.; et al. Dynamic JUNQ inclusion bodies are asymmetrically inherited in mammalian cell lines through the asymmetric partitioning of vimentin. Proc. Natl. Acad. Sci. USA 2014, 111, 8049–8054.
- Rivett, A.J. Intracellular distribution of proteasomes. Curr. Opin. Immunol. 1998, 10, 110–114.
- Wojcik, C.; DeMartino, G.N. Intracellular localization of proteasomes. Int. J. Biochem. Cell Biol. 2003, 35, 579–589.
- Pines, J.; Lindon, C. Proteolysis: Anytime, any place, anywhere? Nat. Cell Biol. 2005, 7, 731–735.
- Laporte, D.; Salin, B.; Daignan-Fornier, B.; Sagot, I. Reversible cytoplasmic localization of the proteasome in quiescent yeast cells. J. Cell Biol. 2008, 181, 737–745.
- Marshall, R.S.; Vierstra, R.D. Proteasome storage granules protect proteasomes from autophagic degradation upon carbon starvation. eLife 2018, 7, e34532.
- Saitoh, Y.; Sawada, H.; Yokosawa, H. High-molecular-weight protease complexes (proteasomes) of sperm of the ascidian, Halocynthia roretzi: Isolation, characterization, and physiological roles in fertilization. Dev. Biol. 1993, 158, 238–244.
- Sawada, H.; Pinto, M.R.; De Santis, R. Participation of sperm proteasome in fertilization of the phlebobranch ascidian Ciona intestinalis. Mol. Reprod. Dev. 1998, 50, 493–498.
- Sawada, H.; Sakai, N.; Abe, Y.; Tanaka, E.; Takahashi, Y.; Fujino, J.; Kodama, E.; Takizawa, S.; Yokosawa, H. Extracellular ubiquitination and proteasome-mediated degradation of the ascidian sperm receptor. Proc. Natl. Acad. Sci. USA 2002, 99, 1223–1228.
- Wada, M.; Kosaka, M.; Saito, S.; Sano, T.; Tanaka, K.; Ichihara, A. Serum concentration and localization in tumor cells of proteasomes in patients with hematologic malignancy and their pathophysiologic significance. J. Lab. Clin. Med. 1993, 121, 215–223.
- Choi, W.H.; Kim, S.; Park, S.; Lee, M.J. Concept and application of circulating proteasomes. Exp. Mol. Med. 2021, 53, 1539–1546.
- Dwivedi, V.; Yaniv, K.; Sharon, M. Beyond cells: The extracellular circulating 20S proteasomes. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166041.
- Sixt, S.U.; Dahlmann, B. Extracellular, circulating proteasomes and ubiquitin-incidence and relevance. Biochim. Biophys. Acta 2008, 1782, 817–823.
- Kulichkova, V.A.; Artamonova, T.O.; Lyublinskaya, O.G.; Khodorkovskii, M.A.; Tomilin, A.N.; Tsimokha, A.S. Proteomic analysis of affinity-purified extracellular proteasomes reveals exclusively 20S complexes. Oncotarget 2017, 8, 102134–102149.
- Tsimokha, A.S.; Zaykova, J.J.; Bottrill, A.; Barlev, N.A. Extracellular proteasomes are deficient in 19S subunits as revealed by iTRAQ quantitative proteomics. J. Cell Physiol. 2017, 232, 842–851.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Breckel, C.A.; Hochstrasser, M. Ubiquitin ligase redundancy and nuclear-cytoplasmic localization in yeast protein quality control. Biomolecules 2021, 11, 1821.
- Hirayama, S.; Sugihara, M.; Morito, D.; Iemura, S.I.; Natsume, T.; Murata, S.; Nagata, K. Nuclear export of ubiquitinated proteins via the UBIN-POST system. Proc. Natl. Acad. Sci. USA 2018, 115, e4199–e4208.
- Kopito, R.R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000, 10, 524–530.
- Miller, S.B.; Ho, C.T.; Winkler, J.; Khokhrina, M.; Neuner, A.; Mohamed, M.Y.; Guilbride, D.L.; Richter, K.; Lisby, M.; Schiebel, E.; et al. Compartment-specific aggregases direct distinct nuclear and cytoplasmic aggregate deposition. EMBO J. 2015, 34, 778–797.
- Escusa-Toret, S.; Vonk, W.I.; Frydman, J. Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat. Cell Biol. 2013, 15, 1231–1243.
- Miller, S.B.; Mogk, A.; Bukau, B. Spatially organized aggregation of misfolded proteins as cellular stress defense strategy. J. Mol. Biol. 2015, 427, 1564–1574.
- Sontag, E.M.; Samant, R.S.; Frydman, J. Mechanisms and functions of spatial protein quality control. Annu. Rev. Biochem. 2017, 86, 97–122.
- Enam, C.; Geffen, Y.; Ravid, T.; Gardner, R.G. Protein quality control degradation in the nucleus. Annu. Rev. Biochem. 2018, 87, 725–749.
- Kaganovich, D.; Kopito, R.; Frydman, J. Misfolded proteins partition between two distinct quality control compartments. Nature 2008, 454, 1088–1095.
- Wendler, P.; Enenkel, C. Nuclear transport of yeast proteasomes. Front. Mol. Biosci. 2019, 6, 34.
- Enenkel, C. Nuclear transport of yeast proteasomes. Biomolecules 2014, 4, 940–955.
- Burcoglu, J.; Zhao, L.; Enenkel, C. Nuclear import of yeast proteasomes. Cells 2015, 4, 387–405.
- Enenkel, C. Proteasome dynamics. Biochim. Biophys. Acta 2014, 1843, 39–46.
- Chowdhury, M.; Enenkel, C. Intracellular dynamics of the ubiquitin-proteasome-system. F1000Research 2015, 4, 367.
- Kinoshita, M.; Hamakubo, T.; Fukui, I.; Murachi, T.; Toyohara, H. Significant amount of multicatalytic proteinase identified on membrane from human erythrocyte. J. Biochem. 1990, 107, 440–444.
- Rivett, A.J.; Palmer, A.; Knecht, E. Electron microscopic localization of the multicatalytic proteinase complex in rat liver and in cultured cells. J. Histochem. Cytochem. 1992, 40, 1165–1172.
- Takeda, K.; Yanagida, M. Regulation of nuclear proteasome by Rhp6/Ubc2 through ubiquitination and destruction of the sensor and anchor Cut8. Cell 2005, 122, 393–405.
- Kalies, K.U.; Allan, S.; Sergeyenko, T.; Kröger, H.; Römisch, K. The protein translocation channel binds proteasomes to the endoplasmic reticulum membrane. EMBO J. 2005, 24, 2284–2293.
- Kaiser, M.L.; Römisch, K. Proteasome 19S RP binding to the Sec61 channel plays a key role in ERAD. PLoS ONE 2015, 10, e0117260.
- Albert, S.; Wietrzynski, W.; Lee, C.W.; Schaffer, M.; Beck, F.; Schuller, J.M.; Salomé, P.A.; Plitzko, J.M.; Baumeister, W.; Engel, B.D. Direct visualization of degradation microcompartments at the ER membrane. Proc. Natl. Acad. Sci. USA 2020, 117, 1069–1080.
- Enenkel, C.; Lehmann, A.; Kloetzel, P.M. Subcellular distribution of proteasomes implicates a major location of protein degradation in the nuclear envelope-ER network in yeast. EMBO J. 1998, 17, 6144–6154.
- Albert, S.; Schaffer, M.; Beck, F.; Mosalaganti, S.; Asano, S.; Thomas, H.F.; Plitzko, J.M.; Beck, M.; Baumeister, W.; Engel, B.D. Proteasomes tether to two distinct sites at the nuclear pore complex. Proc. Natl. Acad. Sci. USA 2017, 114, 13726–13731.
- Nakagawa, T.; Shirane, M.; Iemura, S.; Natsume, T.; Nakayama, K.I. Anchoring of the 26S proteasome to the organellar membrane by FKBP38. Genes Cells 2007, 12, 709–719.
- Fricke, B.; Heink, S.; Steffen, J.; Kloetzel, P.M.; Krüger, E. The proteasome maturation protein POMP facilitates major steps of 20S proteasome formation at the endoplasmic reticulum. EMBO Rep. 2007, 8, 1170–1175.
- Schmidt, O.; Weyer, Y.; Baumann, V.; Widerin, M.A.; Eising, S.; Angelova, M.; Schleiffer, A.; Kremser, L.; Lindner, H.; Peter, M.; et al. Endosome and Golgi-associated degradation (EGAD) of membrane proteins regulates sphingolipid metabolism. EMBO J. 2019, 38, e101433.
- Eisenberg-Lerner, A.; Benyair, R.; Hizkiahou, N.; Nudel, N.; Maor, R.; Kramer, M.P.; Shmueli, M.D.; Zigdon, I.; Cherniavsky Lev, M.; Ulman, A.; et al. Golgi organization is regulated by proteasomal degradation. Nat. Commun. 2020, 11, 409.
- Gorbea, C.; Goellner, G.M.; Teter, K.; Holmes, R.K.; Rechsteiner, M. Characterization of mammalian Ecm29, a 26 S proteasome-associated protein that localizes to the nucleus and membrane vesicles. J. Biol. Chem. 2004, 279, 54849–54861.
- Gorbea, C.; Pratt, G.; Ustrell, V.; Bell, R.; Sahasrabudhe, S.; Hughes, R.E.; Rechsteiner, M. A protein interaction network for Ecm29 links the 26 S proteasome to molecular motors and endosomal components. J. Biol. Chem. 2010, 285, 31616–31633.
- Lee, M.; Liu, Y.C.; Chen, C.; Lu, C.H.; Lu, S.T.; Huang, T.N.; Hsu, M.T.; Hsueh, Y.P.; Cheng, P.L. Ecm29-mediated proteasomal distribution modulates excitatory GABA responses in the developing brain. J. Cell Biol. 2020, 219, e201903033.
- Ibañez-Vega, J.; Del Valle, F.; Sáez, J.J.; Guzman, F.; Diaz, J.; Soza, A.; Yuseff, M.I. Ecm29-dependent proteasome localization regulates cytoskeleton remodeling at the immune synapse. Front. Cell Dev. Biol. 2021, 9, 650817.
- Sarraf, S.A.; Raman, M.; Guarani-Pereira, V.; Sowa, M.E.; Huttlin, E.L.; Gygi, S.P.; Harper, J.W. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 2013, 496, 372–376.
- Livnat-Levanon, N.; Glickman, M.H. Ubiquitin-proteasome system and mitochondria-reciprocity. Biochim. Biophys. Acta 2011, 1809, 80–87.
- Lehmann, G.; Udasin, R.G.; Ciechanover, A. On the linkage between the ubiquitin-proteasome system and the mitochondria. Biochem. Biophys. Res. Commun. 2016, 473, 80–86.
- Azzu, V.; Brand, M.D. Degradation of an intramitochondrial protein by the cytosolic proteasome. J. Cell Sci. 2010, 123, 578–585.
- Chan, N.C.; Salazar, A.M.; Pham, A.H.; Sweredoski, M.J.; Kolawa, N.J.; Graham, R.L.; Hess, S.; Chan, D.C. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 2011, 20, 1726–1737.
- Yoshii, S.R.; Kishi, C.; Ishihara, N.; Mizushima, N. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J. Biol. Chem. 2011, 286, 19630–19640.
- Wiertz, E.J.; Tortorella, D.; Bogyo, M.; Yu, J.; Mothes, W.; Jones, T.R.; Rapoport, T.A.; Ploegh, H.L. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 1996, 384, 432–438.
- Ng, W.; Sergeyenko, T.; Zeng, N.; Brown, J.D.; Römisch, K. Characterization of the proteasome interaction with the Sec61 channel in the endoplasmic reticulum. J. Cell Sci. 2007, 120, 682–691.
- Wu, X.; Rapoport, T.A. Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol. 2018, 53, 22–28.
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018, 28, r170–r185.
- Sun, Z.; Brodsky, J.L. Protein quality control in the secretory pathway. J. Cell Biol. 2019, 218, 3171–3187.
- Montenegro-Venegas, C.; Fienko, S.; Anni, D.; Pina-Fernández, E.; Frischknecht, R.; Fejtova, A. Bassoon inhibits proteasome activity via interaction with PSMB4. Cell Mol. Life Sci. 2021, 78, 1545–1563.
- Bingol, B.; Schuman, E.M. Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature 2006, 441, 1144–1148.
- Bingol, B.; Sheng, M. Deconstruction for reconstruction: The role of proteolysis in neural plasticity and disease. Neuron 2011, 69, 22–32.
- Bingol, B.; Wang, C.-F.; Arnott, D.; Cheng, D.; Peng, J.; Sheng, M. Autophosphorylated CaMKIIα acts as a scaffold to recruit proteasomes to dendritic spines. Cell 2010, 140, 567–578.
- Ehlers, M.D. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 2003, 6, 231–242.
- Otero, M.G.; Alloatti, M.; Cromberg, L.E.; Almenar-Queralt, A.; Encalada, S.E.; Pozo Devoto, V.M.; Bruno, L.; Goldstein, L.S.; Falzone, T.L. Fast axonal transport of the proteasome complex depends on membrane interaction and molecular motor function. J. Cell Sci. 2014, 127, 1537–1549.
- Liu, K.; Jones, S.; Minis, A.; Rodriguez, J.; Molina, H.; Steller, H. PI31 Is an adaptor protein for proteasome transport in axons and required for synaptic development. Dev. Cell 2019, 50, 509–524.e10.
- Shi, L.; Liu, B.; Shen, D.-D.; Yan, P.; Zhang, Y.; Tian, Y.; Hou, L.; Jiang, G.; Zhu, Y.; Liang, Y.; et al. A tumor-suppressive circular RNA mediates uncanonical integrin degradation by the proteasome in liver cancer. Sci. Adv. 2021, 7, eabe5043.
- Ramachandran, K.V.; Fu, J.M.; Schaffer, T.B.; Na, C.H.; Delannoy, M.; Margolis, S.S. Activity-dependent degradation of the nascentome by the neuronal membrane proteasome. Mol. Cell 2018, 71, 169–177.e6.
- Ramachandran, K.V.; Margolis, S.S. A mammalian nervous-system-specific plasma membrane proteasome complex that modulates neuronal function. Nat. Struct. Mol. Biol. 2017, 24, 419–430.
- Türker, F.; Cook, E.K.; Margolis, S.S. The proteasome and its role in the nervous system. Cell Chem. Biol. 2021, 28, 903–917.
- Sahu, I.; Mali, S.M.; Sulkshane, P.; Xu, C.; Rozenberg, A.; Morag, R.; Sahoo, M.P.; Singh, S.K.; Ding, Z.; Wang, Y.; et al. The 20S as a stand-alone proteasome in cells can degrade the ubiquitin tag. Nat. Commun. 2021, 12, 6173.
- Shibahara, T.; Kawasaki, H.; Hirano, H. Identification of the 19S regulatory particle subunits from the rice 26S proteasome. Eur. J. Biochem. 2002, 269, 1474–1483.
- Kikuchi, J.; Iwafune, Y.; Akiyama, T.; Okayama, A.; Nakamura, H.; Arakawa, N.; Kimura, Y.; Hirano, H. Co- and post-translational modifications of the 26S proteasome in yeast. Proteomics 2010, 10, 2769–2779.
- Kimura, A.; Kato, Y.; Hirano, H. N-myristoylation of the Rpt2 subunit regulates intracellular localization of the yeast 26S proteasome. Biochemistry 2012, 51, 8856–8866.
- Kimura, A.; Kurata, Y.; Nakabayashi, J.; Kagawa, H.; Hirano, H. N-Myristoylation of the Rpt2 subunit of the yeast 26S proteasome is implicated in the subcellular compartment-specific protein quality control system. J. Proteom. 2016, 130, 33–41.
- Kimura, Y.; Saeki, Y.; Yokosawa, H.; Polevoda, B.; Sherman, F.; Hirano, H. N-Terminal modifications of the 19S regulatory particle subunits of the yeast proteasome. Arch. Biochem. Biophys. 2003, 409, 341–348.
- Zong, C.; Gomes, A.V.; Drews, O.; Li, X.; Young, G.W.; Berhane, B.; Qiao, X.; French, S.W.; Bardag-Gorce, F.; Ping, P. Regulation of murine cardiac 20S proteasomes: Role of associating partners. Circ. Res. 2006, 99, 372–380.
- Wang, X.; Chen, C.F.; Baker, P.R.; Chen, P.L.; Kaiser, P.; Huang, L. Mass spectrometric characterization of the affinity-purified human 26S proteasome complex. Biochemistry 2007, 46, 3553–3565.
- Thinon, E.; Serwa, R.A.; Broncel, M.; Brannigan, J.A.; Brassat, U.; Wright, M.H.; Heal, W.P.; Wilkinson, A.J.; Mann, D.J.; Tate, E.W. Global profiling of co- and post-translationally N-myristoylated proteomes in human cells. Nat. Commun. 2014, 5, 4919.
- Farazi, T.A.; Waksman, G.; Gordon, J.I. The biology and enzymology of protein N-myristoylation. J. Biol. Chem. 2001, 276, 39501–39504.
- Johnson, D.R.; Bhatnagar, R.S.; Knoll, L.J.; Gordon, J.I. Genetic and biochemical studies of protein N-myristoylation. Annu. Rev. Biochem. 1994, 63, 869–914.
- Rajala, R.V.; Datla, R.S.; Moyana, T.N.; Kakkar, R.; Carlsen, S.A.; Sharma, R.K. N-myristoyltransferase. Mol. Cell Biochem. 2000, 204, 135–155.
- Chen, L.; Zhang, Y.; Shu, X.; Chen, Q.; Wei, T.; Wang, H.; Wang, X.; Wu, Q.; Zhang, X.; Liu, X.; et al. Proteasome regulation by reversible tyrosine phosphorylation at the membrane. Oncogene 2021, 40, 1942–1956.
- Kamps, M.P.; Buss, J.E.; Sefton, B.M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc. Natl. Acad. Sci. USA 1985, 82, 4625–4628.
- Lam, S.S.; Martell, J.D.; Kamer, K.J.; Deerinck, T.J.; Ellisman, M.H.; Mootha, V.K.; Ting, A.Y. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 2015, 12, 51–54.
- Marmor-Kollet, H.; Siany, A.; Kedersha, N.; Knafo, N.; Rivkin, N.; Danino, Y.M.; Moens, T.G.; Olender, T.; Sheban, D.; Cohen, N.; et al. Spatiotemporal proteomic analysis of stress granule disassembly using APEX reveals regulation by SUMOylation and links to ALS pathogenesis. Mol. Cell 2020, 80, 876–891.e6.
- Liu, Q.; Zheng, J.; Sun, W.; Huo, Y.; Zhang, L.; Hao, P.; Wang, H.; Zhuang, M. A proximity-tagging system to identify membrane protein-protein interactions. Nat. Methods 2018, 15, 715–722.
- Yasuda, S.; Tsuchiya, H.; Kaiho, A.; Guo, Q.; Ikeuchi, K.; Endo, A.; Arai, N.; Ohtake, F.; Murata, S.; Inada, T.; et al. Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 2020, 578, 296–300.
- Liu, X.; Xiao, W.; Zhang, Y.; Wiley, S.E.; Zuo, T.; Zheng, Y.; Chen, N.; Chen, L.; Wang, X.; Zheng, Y.; et al. Reversible phosphorylation of Rpn1 regulates 26S proteasome assembly and function. Proc. Natl. Acad. Sci. USA 2020, 117, 328–336.
- Zavodszky, E.; Peak-Chew, S.Y.; Juszkiewicz, S.; Narvaez, A.J.; Hegde, R.S. Identification of a quality-control factor that monitors failures during proteasome assembly. Science 2021, 373, 998–1004.
- Wang, X.; Cimermancic, P.; Yu, C.; Schweitzer, A.; Chopra, N.; Engel, J.L.; Greenberg, C.; Huszagh, A.S.; Beck, F.; Sakata, E.; et al. Molecular details underlying dynamic structures and regulation of the human 26S proteasome. Mol. Cell Proteom. 2017, 16, 840–854.
- Tomko, R.J., Jr.; Hochstrasser, M. The intrinsically disordered Sem1 protein functions as a molecular tether during proteasome lid biogenesis. Mol. Cell 2014, 53, 433–443.
- Fu, A.; Cohen-Kaplan, V.; Avni, N.; Livneh, I.; Ciechanover, A. p62-containing, proteolytically active nuclear condensates, increase the efficiency of the ubiquitin-proteasome system. Proc. Natl. Acad. Sci. USA 2021, 118, e2107321118.
- Katayama, H.; Kogure, T.; Mizushima, N.; Yoshimori, T.; Miyawaki, A. A sensitive and quantitative technique for detecting autophagic events based on lysosomal delivery. Chem. Biol. 2011, 18, 1042–1052.
- Martinez-Fonts, K.; Davis, C.; Tomita, T.; Elsasser, S.; Nager, A.R.; Shi, Y.; Finley, D.; Matouschek, A. The proteasome 19S cap and its ubiquitin receptors provide a versatile recognition platform for substrates. Nat. Commun. 2020, 11, 477.
- Tomita, T.; Hirayama, S.; Sakurai, Y.; Ohte, Y.; Yoshihara, H.; Saeki, Y.; Hamazaki, J.; Murata, S. Specific modification of aged proteasomes revealed by tag-exchangeable knock-in mice. Mol. Cell Biol. 2019, 39, e00426-18.
- de Almeida, M.; Hinterndorfer, M.; Brunner, H.; Grishkovskaya, I.; Singh, K.; Schleiffer, A.; Jude, J.; Deswal, S.; Kalis, R.; Vunjak, M.; et al. AKIRIN2 controls the nuclear import of proteasomes in vertebrates. Nature 2021, 599, 491–496.
- Gu, Z.C.; Wu, E.; Sailer, C.; Jando, J.; Styles, E.; Eisenkolb, I.; Kuschel, M.; Bitschar, K.; Wang, X.; Huang, L.; et al. Ubiquitin orchestrates proteasome dynamics between proliferation and quiescence in yeast. Mol. Biol. Cell 2017, 28, 2479–2491.
- Jongkamonwiwat, N.; Ramirez, M.A.; Edassery, S.; Wong, A.C.Y.; Yu, J.; Abbott, T.; Pak, K.; Ryan, A.F.; Savas, J.N. Noise exposures causing hearing loss generate proteotoxic stress and activate the proteostasis network. Cell Rep. 2020, 33, 108431.
- Xie, F.; Su, P.; Pan, T.; Zhou, X.; Li, H.; Huang, H.; Wang, A.; Wang, F.; Huang, J.; Yan, H.; et al. Engineering extracellular vesicles enriched with palmitoylated ACE2 as COVID-19 therapy. Adv. Mater. 2021, 49, e2103471.
- Huang, R.; Han, M.; Meng, L.; Chen, X. Transcriptome-wide discovery of coding and noncoding RNA-binding proteins. Proc. Natl. Acad. Sci. USA 2018, 115, e3879–e3887.
- Castello, A.; Fischer, B.; Eichelbaum, K.; Horos, R.; Beckmann, B.M.; Strein, C.; Davey, N.E.; Humphreys, D.T.; Preiss, T.; Steinmetz, L.M.; et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 2012, 149, 1393–1406.
- Quinodoz, S.A.; Jachowicz, J.W.; Bhat, P.; Ollikainen, N.; Banerjee, A.K.; Goronzy, I.N.; Blanco, M.R.; Chovanec, P.; Chow, A.; Markaki, Y.; et al. RNA promotes the formation of spatial compartments in the nucleus. Cell 2021, 184, 5775–5790.e30.
- Uriarte, M.; Sen Nkwe, N.; Tremblay, R.; Ahmed, O.; Messmer, C.; Mashtalir, N.; Barbour, H.; Masclef, L.; Voide, M.; Viallard, C.; et al. Starvation-induced proteasome assemblies in the nucleus link amino acid supply to apoptosis. Nat. Commun. 2021, 12, 6984.
- Brehm, A.; Liu, Y.; Sheikh, A.; Marrero, B.; Omoyinmi, E.; Zhou, Q.; Montealegre, G.; Biancotto, A.; Reinhardt, A.; Almeida de Jesus, A.; et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J. Clin. Investig. 2015, 125, 4196–4211.
- Torrelo, A. CANDLE syndrome as a paradigm of proteasome-related autoinflammation. Front. Immunol. 2017, 8, 927.
This entry is offline, you can click here to edit this entry!
