Controlled release of substance from polyelectrolyte microcapsules is a triggered degradation of the microcapsule membrane that is extensive enough to release the contained substances out into the environment. Membrane degradation can be a result of enzymatic digestion, ultrasound or light exposure, heating, application of a magnetic field, pH or ionic strength changes in the solution or bacteria-mediated processes. This technology can be used for the targeted release of drugs, and for the development of self-healing materials and new generation pesticides.
- polyelectrolyte microcapsules
- decapsulation
- controlled release
1. Introduction
Compared to other types of encapsulations, polyelectrolyte microcapsules have one of the main advantages—a variety of methods for the controlled release of the encapsulated substance. Due to the variability in the composition of a PMC shell, there are many ways to achieve the controlled release of the contained macromolecules. A variety of methods for the controlled release of substances from a PMC will allow the delivery of drugs to the target organ and release it locally, to create self-healing materials, pesticides (gradual release of pesticides) and genomic editing tools. Thus, the systematization of the results obtained over the past few decades on the controlled release of substances from polyelectrolyte microcapsules, will clarify the perception of the researcher in this field of study.
2. History
In the early 1990s, Decher and coauthors [1,2] were the first ones to demonstrate that it was possible to modify a surface with a layer-by-layer (LbL) technique, which later became widely used. A step-by-step buildup of a multilayer film is mediated by the formation of ionic interactions between oppositely charged areas of polyelectrolytes. Starting in 1998 [3], the LbL self-assembly technique was used as a novel tool for nano- and micro-encapsulation. In this method, multilayer polyelectrolyte films were built around different particles by the consecutive adsorption of oppositely charged polyelectrolytes via their electrostatic attraction, together with other association-mediating interactions, such as hydrogen bond formation, hydrophobic effects, charge transfer reactions, etc. Efficient microencapsulation of biologically or chemically active substances—drugs, proteins, vitamins, flavors, gas bubbles and even whole living cells—is getting more and more important for numerous purposes in the fields of biochemistry, pharmaceutics, cosmetology and catalysis [4].
The control over the microcapsule shell composition allows the creation of polyelectrolyte microcapsules (PMCs) that are able to release the encapsulated drug in response to some trigger. Decapsulation is necessary for targeted therapy development, which allows the delivery and release of a drug to the organs or tissues of interest. Such an approach minimizes the side effects of the drug and reduces the patient’s rehabilitation duration. Moreover, gradual release of small amounts of the drug would allow its effect in the organism to be prolonged, for example, gradual release of insulin into the circulation. This technology is also used for the development of self-healing materials, pesticides and for cell culture cultivation.
The first triggered decomposition of PMCs was shown in the work of C. Schüler and F. Caruso (2001) [5]. They created polyelectrolyte capsules using DNA/spermidine. It is known that DNA/spermidine interactions weaken in solutions with higher ionic strength. Thus, after DNA/spermidine capsules had been placed into the 5M salt solution, the multilayers dissolved, leading to the degradation of the capsule.
Microcapsule shell degradation, in response to the pH change of the surrounding solution, appeared to be the newly developed way of decapsulation in the history of PMCs. In this case, microcapsules must consist of strong and weak polyelectrolytes, and when the pH of the solution becomes higher (in the case of polybase) or lower (in the case of polyacid) than the pKa of the weak polyelectrolyte, polyelectrolytes lose their charge, which results in capsule degradation (2002) [6].
Later on, numerous ways of decapsulation were discovered: heating (2002) [7], light-sensitive degradation (2004) [8], magnetic field application (2005) [9], microwave radiation (2006) [10], ultrasound application (2006) [11] and even bacterial spore germination (2020) [12]. Today, the most widespread technique for decapsulation is enzymatic digestion. In this case, microcapsules are created with the use of biodegradable polyelectrolytes, such as polysaccharides, polypeptides or polynucleotides [13].
3. The Ways of Encapsulated Substance Release
One of the essential questions in using PMCs as microcontainers for targeted drug delivery is the release of the encapsulated substance. Since both physical and chemical media factors strongly affect the polyelectrolyte shell of a PMC, release of the drug from the capsule can be initiated by acidity, ionic strength or polarity of the solution, glucose concentration, light, ultrasound, magnetic field application, redox state of the solution, enzymatic reactions or bacterial growth (Table 1).
Table 1. Methods for controlled release of substances from polyelectrolyte microcapsules.
|
The Initiator of Substance Release |
Microcapsule’s Constitution (First Layer/Second Layer)Number of Repeats |
Released Substance |
Citation |
|
Acidity of the solution (pH) |
BSA/(PAH/PSS)5 |
Doxorubicin |
[14] |
|
Ionic strength of the solution |
(PSS/PAH)9/PSS |
Fluorescein |
[15] |
|
Glucose |
(PAD/PSS)6 |
FITC-BSA |
[16] |
|
(Mannan/PAH-BOH)7 |
TRITC-BSA |
[17] |
|
|
Light |
(PSS/PDADMAC)5/PSS/DDAB/SiO2-TiO2 |
Phenol red |
[18] |
|
MF/(PSS/PAH)4PSS |
FITC-dextran |
[19] |
|
|
(PAH-BP/PSS)4 |
Rhodamine B |
[20] |
|
|
(PSS/PAH)2-Ag-(PSS/PAH)2 |
- |
[21] |
|
|
Bt-SiO2/PEI/PSS/PEI/PSS Bt-TiO2/PEI/PSS/ PEI/PSS |
Benzotriazole |
[22] |
|
|
(PDADMAC/Au/PSS)4 |
Alexa Fluor 555 dextran |
[23] |
|
|
(PAH/PMA)4 /NR |
Nimbin and Doxorubicin |
[24] |
|
|
Ultrasound |
(PSS/PAH)2/Ag/(PSS/PAH)2 (PSS/PAH)2/Ag/(PSS/PAH)8 |
FITC-PAH |
[25] |
|
(PAH/DS)3/AgNPs/(PAH/DS) |
FITC-dextran |
[26] |
|
|
(PSS/PAH)4 (AuNP/PAH)4 |
FITC-dextran |
[27] |
|
|
(PAH/PSS)2(PAH/Fe3O4)4(PAH/PSS)2 |
FITC-dextran |
[11] |
|
|
Magnetic field |
(PAH/ Fe3O4 NPs)4 |
Insulin |
[28] |
|
(PSS/PAH)5/Pd |
Phenol red |
[29] |
|
|
(PDADMAC/PSS)6 |
FITC-dextran |
[30] |
|
|
(PSS/PAH)/(PSS/P(Am-DDA)/ NPs/ (PAH)(PSS/PAH)2 |
Cascade Blue-labelled dextran |
[31] |
|
|
Redox |
(PVPON/PMASH)4 |
FITC-transferrin |
[32] |
|
(PSS-Rh/PAH)2/(PSS/PAH)3 (PSS-Rh/PAH)2/(PSS/PAH)3/PSS (PSS-Rh/PAH)2/(PGA/PLL)4 (PSS-Rh/PAH)2/(PGA/PLL)4/PGA |
9-amino-acid peptide KP9 |
[33] |
|
|
Enzymatic digestion |
(PSS/P(HPMA-DMAE))4 |
FITC-dextran |
[34] |
|
(DS/PLA)4 |
Rhodamine isothiocyanate |
[35] |
|
|
(CT/DS)3 |
FITC-albumin |
[36] |
|
|
(CHI/ALG)5 |
Indomethacin |
[37] |
|
|
Osmotic pressure |
(Dex-HEMA-DMAEMA)/(PSS/PAH)3 |
FITC-dextran |
[38] |
|
(Dex-HEMA-DMAEMA)/(PSS/PAH)3 |
TRITC-dextran |
[39] |
|
|
Spore germination |
(spore forms of Bacillus subtilis)/(PSS/PAH)3 |
FITC-dextran |
[12] |
3.1. Acidity of the Solution (pH)
The proof of principle of controlled, pH-dependent decapsulation was shown in the study of Shen et al. [14]. The authors used PMCs containing BSA gel. Such microcapsules were obtained by heating the PMCs with BSA up to 80 °С; this led to the formation of polyampholitic gel, which changed its charge in response to the change in the pH of the solution. The efficacy of the method was proved by the example of doxorubicin encapsulation: at pH > 4.8 doxorubicin was charged positively, BSA was charged negatively and an electrostatic interaction between them emerged. The interaction between BSA and doxorubicin led to the loading of the latter into the capsule. When the pH of the solution was decreased, BSA changed its charge to positive, which disrupted its electrostatic interactions with and led to the decapsulation of doxorubicin. In theory, this method can be used for reciprocal situations as well: cargo load at low pH, if the cargo has a negative charge, and decapsulation of the cargo at high pH, when BSA could change its charge to negative [14].
3.2. Ionic Strength of the Solution
In contrast to pH-controlled decapsulation, drug release in response to the ionic strength of the solution can happen for PMCs containing both weak and strong polyelectrolytes. Antipov et al. [15] showed that the mechanism of ionic strength-mediated decapsulation is quite similar to the pH-controlled one: with an increase in ionic strength of the solution, free energy of interactions within the polyelectrolyte layers decreases leading to higher permeability of the capsules and to the release of fluorescein from the PMC ((PSS/PAH)9/PSS). In another study [40], it was reported that an increase in the ionic strength of the solution resulted in the generation of local defects in the capsule and, as a result, in increased shell permeability. However, lack of linear dependence between the permeability of the capsule, salt concentration in the solution and the inability to use this approach for controlled decapsulation in vivo, resulted in a significant limitation to its applications.
3.3. Glucose Content
In the study of De Geest et al. [16], the authors created polyelectrolyte microcapsules using a glucose-sensitive polymer. Polystyrene sulphonate and copolymer of dimethylaminoethyl acrylate and 3-acrylaminophenylboronic acid were used as the main components of the capsule shell in this work. The glucose-responsive component here was a derivative of the phenylboronic acid—it could form complexes with glucose, leading to the shift in chemical equilibrium towards charged acid molecules. This resulted in increased electrostatic interactions, repulsion of microcapsule layers and capsule shell degradation [16].
Levy et al. [17] created another system based on forming ester bonds between polysaccharides and phenylboronic acid derivatives. In this case, the PMC shell contained complexes of polyacrylic acid with hemisulphate of aminophenylboronic acid, which bound with mannans through ester bonds. It was observed that the increase in simple carbohydrates (fructose, glucose, mannose and galactose) led to the disruption of the microcapsule shell. It likely occurred as a result of simple carbohydrates replacing mannans in complexes with the phenylboronic acid derivatives, which led to destabilization of the capsule shell layers and capsule degradation [17].
3.4. Light Exposure
Light exposure appears to be a convenient way to control decapsulation (Figure 1). For instance, Katagiri et al. [18] created UV-sensitive PMCs with the shell consisting of polystyrenesulfonate and polydiallyldimethylammonium layers coated with a lipid bilayer and SiO2-TiO2 oxide system. In this study it was shown that encapsulated low molecular weight substances could be released by UV irradiation due to TiO2 photocatalytic activity, which promoted degradation of the polyelectrolyte layers of the capsule. The rate of capsule disruption and, as a result, the rate of the cargo release were regulated by the SiO2:TiO2 ratio in the outer layer of the capsule [18]. Another example of UV-controlled microcapsules was demonstrated in the study of Koo et al. [19]. The authors of this work developed PMCs with photoacid generators (PAGs) in the outer layer of the capsule shell. After UV irradiation of such capsules the PAGs released protons into the surrounding solution, which led to a local decrease in pH. Thus, long UV exposure, caused by maintaining low pH values, resulted in the swelling and disruption of the PMCs [19].
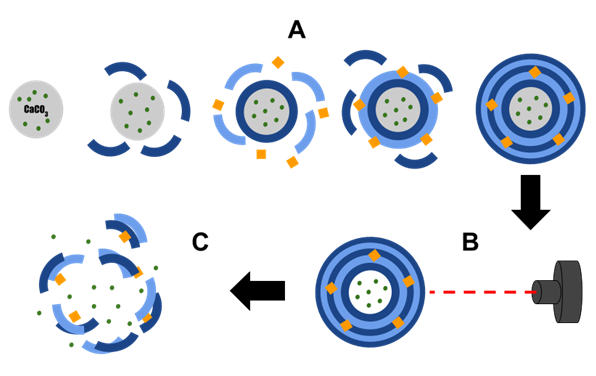
Figure 1. Diagram showing the effect of light on substance release from polyelectrolyte microcapsules. A—formation of polyelectrolyte microcapsules with metal particles; B—irradiation of microcapsules with light; C—destruction of microcapsules after light irradiation.
The work of Park et al. [20] is another example of UV application for controlled decapsulation. Authors used photosensitive benzophenone-modified polystyrenesulphonate (PSS) and polyallylamine (PAH) for the microcapsule shell assembly. After UV exposure, the proton in the C–H bond in the polymer layer was easily abstracted by excited benzophenone, leading to the formation of a new C–C bond between the polymers of the shell. It was demonstrated that the time of UV irradiation affected the extent of cross-linking and the pore size, which allowed the fine-tuning of the rate of the drug release from the microcapsule [20].
Other than UV light, light-controlled decapsulation can also be implemented via use of near-infrared (IR) band frequencies (700–900 nm). In contrast to UV light, IR does not have any negative effects on the living cells and can penetrate quite deeply into an organism’s tissues. In the work of Skirtach et al. [21], the authors created microcapsules consisting of PAH and PSS and containing either silver nanoparticles or IR-absorbing dyes. It was reported that after exposure to laser light of IR band frequencies, multiple gaps in the PMC shell appeared and the capsule’s cargo was released [21]. The same phenomenon was demonstrated by Skorb et al. [22]. In this work, they created capsules consisting of PSS and polyethylenimine (PEI), which were modified by silver nanoparticles. IR laser irradiation of such capsules resulted in the formation of pores large enough for anticorrosion cargo compound release.
In the study of Skirtach et al. [23], the authors used aggregates of gold nanoparticles to create IR-controlled PMCs. They incorporated gold nanoparticle aggregates into the microcapsule shell, which consisted of polydiallyldimethylammonium (PDADMA) and PSS. As a result, PMCs with the peak of the light absorption in the IR part of the spectrum were obtained. Gold particles absorbed the IR light and heated up, leading to the local melting of polymer layers and an increase in their permeability. At the same time, after the light exposure was stopped, the permeability of the PMC shell returned to its initial values [23].
This decapsulation method was extensively used in the application of PMCs to photothermal cancer therapy. It allowed substances to act on cancerous cells in two different ways—via a therapeutic chemical compound and via microcapsule heating. A recent study demonstrated the considerable potential of IR-absorbing microcapsules and laser-triggered decapsulation for applications in anticancer therapy [24]. Authors created PMCs containing two drugs: hydrophilic doxorubicin and hydrophobic anticancer drug, nimbin [24]. Decapsulation of these PMCs was triggered by near-infrared (NIR) light exposure and, in contrast to other studies, this work demonstrated the ability to use a low power laser (0.5 W/cm2) for decapsulation initiation.
3.5. Ultrasound
Ultrasound-based tools are widely used for medical applications; therefore, ultrasound use for controlled drug release from PMCs appeared to be very promising since its safety and overall effect on the human organism are well described. In general, ultrasound affects the permeability of microcapsules through the cavitation effect in the liquid medium, which occurs when applied ultrasound waves have frequencies of 20 kHz or greater. Ultrasound waves cause the formation of microbubbles from the gases dissolved in the medium. Longer ultrasound exposure leads to microbubble oscillation and collapse, which is called the cavitation phenomenon. Bubble collapse, in turn, causes the redistribution of energy in the media and the generation of additional shear force between liquid layers [41]. As a result of all these processes, the PMC shell can be disturbed or destroyed. The majority of works in this field were performed based on PMCs, which consisted of PSS as a polyanion and PAH as a polycation. One of the first works in the field is the study by Skirtach et al. [25]. Using FITC-dextran as a cargo, the authors demonstrated the possibility of using ultrasound for controlled drug release and, moreover, they proved that the sensitivity of PMCs and the rate of their destruction could be increased by adding silver nanoparticles to the microcapsule shell. Similar results with the release of FITC-dextran in decapsulation were obtained in the study of Anandhakumar et al. [26], which used PAH/DS (dextran sulphate) capsules with incorporated silver particles. Another early work in the field of ultrasound-sensitive PMCs was the work by Shchukin et al. [11]. The authors used the same polycations and polyanions for PMC assembly, but they incorporated iron oxide (II-III) Fe3O4 into one of the middle layers. The addition of iron oxide increased the susceptibility of microcapsules to ultrasound exposure. The strongest effect on ultrasound susceptibility of PMCs was reported in the study of Kolesnikova et al. [42], where the authors used zinc oxide particles in the formulation of PAH/PSS capsules.
It is worth mentioning that the incorporation of metal into the capsule shell did not always affect its susceptibility to ultrasound in this way. For instance, De Geest et al. [27] created two types of PMCs: one consisted only from PSS and PAH, while another contained PAH and gold nanoparticles carrying carboxyl groups on their surface. When two types of microcapsules were compared using their sensitivity to ultrasound, it turned out that PMCs containing gold nanoparticles were more resistant to ultrasound exposure. Such an effect possibly occurred due to polyanionic carboxyl groups, which made the multilayered shell of the microcapsule more rigid [27].
3.6. Magnetic Field
Another example of a trigger for the controlled substance release from PMCs is the application of a magnetic field. One of the main advantages of this method is a high permeability of biological tissues to magnetic fields. In one of the pioneering works about this topic, Caruso et al. [43] created PMCs that not only contained polyelectrolytes in the shell, but also had a polystyrene core. However, the proof of concept of magnetically triggered microcapsule decapsulation was first demonstrated in 2005 by Lu et al. [9]. For this purpose, the authors used microcapsules carrying FITC-dextran as a cargo. The shell of these microcapsules consisted of PAH and PSS with incorporated cobalt nanoparticles, which were coated with gold. After the application of a magnetic field, nanoparticles began to rotate with a frequency corresponding to the frequency of oscillating magnetic field. Thus, these particles destabilized adjacent polyelectrolyte layers and increased the permeability of the PMC shell. At the same time, it was shown that the increase in permeability has its limits, which were imposed by the rate of nanoparticle rotation—if the frequency of the magnetic field became too high, nanoparticles were not able to rotate with the same corresponding rate, and the permeability increase was minimal. Later on, the possible application of capsules containing iron oxide nanoparticles was reported in the study of Zheng et al. [28]. The authors optimized conditions of insulin loading into magnetoresponsive PMCs and demonstrated magnetically controlled decapsulation, thus suggesting the use of this technology in diabetes therapy.
For targeted drug delivery implementation, it is important to create microcapsules with low permeability that can hold compounds with a low molecular weight. Such microcapsules became the main topic of the research carried out by Katagiri et al. [29]. This group developed improved magnetoresponsive PMCs containing Fe3O4 and coated with a lipid bilayer. Such microcapsules can be used for the transportation of substrates with different molecular weights. When a magnetic field was applied, magnetic nanoparticles Fe3O4 increased the permeability of the lipid bilayer of the capsule via particle heating and not through their movements [29].
At present, there have been PMCs developed containing magnetic particles both in the shell and in the core of the microcapsule. Hu et al. [30] created microcapsules containing iron pentacarbonyl Fe(CO)5. Microcapsules were prepared using the layer-by-layer method. First, the magnetoresponsive core was created from iron pentacarbonyl and silicon dioxide, then polyelectrolytes were applied in layers. In the end, silicon dioxide was washed out of the capsules using sodium hydroxide solution. It was shown in this study that microcapsules constructed in such a way were distorted in response to the magnetic field application, which led to the cargo release from the capsule. In this case the fine-tuning of decapsulation was possible by changing the strength of the magnetic field: the stronger the field was, the higher the decapsulation rate was [30].
A similar technique for the fine-tuning of magnetoresponsive microcapsules was developed by Carregal-Romero et al. [31]. The authors created microcapsules containing iron oxide magnetic particles shaped as cubes, which increased their ability to heat up under the influence of the magnetic field. Another way to regulate decapsulation of magnetoresponsive capsules is based on the alteration of the magnetic particle concentration in the shell of a PMC [36]. The higher the concentration, the higher the heat capacity is, and, in turn, higher temperatures can be reached when a magnetic field is applied. Local temperature increases in the shell of the capsule led to capsule structure damage and, as a consequence, to cargo release [44].
All in all, approaches for decapsulation of magnetoresponsive microcapsules can be divided into two categories. In the first one, an alternating magnetic field is used to increase mobility and temperature of magnetic particles [9,45,46], which leads to partial or total destruction of the capsule shell. This approach caused local heating, which could be potentially harmful for healthy tissues and organs, thus limiting its use in clinical applications. The second approach is based on the use of a constant magnetic field, which could be enough for capsule deformation without them heating up [47–48]. However, this way of decapsulation usually requires fabricating microcapsules through an emulsification process, which, in turn, leads to an increase in the size of the resulting microcapsules, up to dozens or even several hundreds of micrometers. Such a large capsule size significantly limits opportunities to use this approach in targeted drug delivery.
3.7. Redox
Redox potential is varied across cellular organelles, and this feature can be used to develop PMCs that are sensitive to this kind of stimuli. One of the first works in this field was dedicated to the development of PMCs, which, in addition to polyelectrolyte compounds, contained cysteine residues in the shell [49]. When put in a solution containing DMSO, disulfide bonds were formed between cysteines, which increased the stability of the capsule shell under low pH values. However, if such capsules were placed in the reducing environment with TCEP, disulfide bonds were disrupted, and the microcapsule shell became susceptible to the action of the acidic pH. Such a system allowed a double-triggered release, which was responsive to both redox potential and the pH of the environment [49].
In another study PMCs were created that released their cargo only in response to the reducing potential of the surrounding solution. Zelikin et al. [32] created microcapsules based on polyvinylpyrrolidone and polymethacrylic acid, modified by thiol groups. Microcapsules were then placed in a strong oxidizing environment to form disulfide bonds between thiol groups. The authors demonstrated that when a reducing agent, such as dithiothreitol (DTT), was applied to these microcapsules, the shell became gradually degraded, releasing the cargo from the capsule [32].
One outstanding example of the use of such PMCs is their possible application as peptide vaccine carriers. In an in vitro study by Rose et al. [33], it was reported that PMCs, loaded with polypeptides, could persistently present in the circulatory system of a human and be engulfed by antigen-presenting cells (APCs). The environment within the cells was reducing, which allowed the release of the cargo inside the cells in response to the change in redox potential. APCs, in turn, became able to present cargo peptides within the main histocompatibility complexes (MHC) on their surface [33].
3.8. Enzymatic Digestion
One of the alternative methods of decapsulation is enzymatic digestion of the shell of the PMC. This may be a convenient way to deliver a drug if it should act inside the cell (as it is in the case of oligonucleotides and proteins). The first example of enzymatically triggered capsules was published by De Geest et al. [34]. The authors developed capsules with two different types of shell: the first consisted of polyarginine and dextran sulphate, and the second consisted of poly(hydroxypropylmethacrylamide dimethylaminoethyl) and PSS. These capsules were then added to the African green monkey kidney cell culture. It was observed that added PMCs were actively engulfed by the cells, and the shell of the capsules was enzymatically degraded, releasing the loaded substance [34]. Such capsules were later investigated using in vivo experiments with mice [35].
One more example of enzymatically driven decapsulation of PMCs was developed in the study of Itoh et al. [36]. The authors obtained microcapsules based on chitosan and sulphate dextran, and used chitosanase as a decapsulation triggering agent. The shell degradation and controlled cargo release was demonstrated after addition of the enzyme to the capsules. Based on these results, the authors decided to go further and try to optimize the system in such a way that the drug release happened slower (so called ‘sustained release’ of the drug). To do this, they added an additional outer layer of positively charged chitosan. In the buffer (pH 5.6) chitosanase (pI 9.3) was also positively charged, thus, the interaction between the enzyme and the modified capsules was weakened, due to the static repulsion [36].
Most of the works in the field of enzymatically controlled capsule degradation include the use of two lytic enzymes, which are present in the human organism: pepsin (in the stomach) and hyaluronidase (in salivary and seminal glands). For pepsin-controlled capsule development, chitosan and poly(2-acrylamido-2-methylpropanesulphonic) acid were used. It was reported that pepsin action led to the degradation of the PMC shell and to the release of the encapsulated indomethacin [37]. Hyaluronidase-responsive PMCs contain the residues of hyaluronic acid [50,51] or chondroitin sulphate [52] in their shells.
As it was shown by Cardoso et al. [53], enzymatic digestion of capsules could be driven not only by action of lytic enzymes from the outside solution, but also by the action of encapsulated enzymes from within the capsule itself. One of the examples of such capsules is the case of the capsules created from chitosan and hyaluronic acid, which are described above. Together with the drug, hyaluronidase was placed into the hollow of the capsule for the triggered release of the capsule shell [53]. One more example of a similar system is the type of capsules described in the work of Borodina et al. [54]. In this work, investigators created a capsule shell consisting of poly-L-arginine and poly-L-aspartic acid. As a decapsulation agent deposited inside the capsule authors used a mix of lytic enzymes from Streptomyces griseus called Pronase. It was also shown that the fine-tuning of the capsule degradation rate could be achieved through the alteration of the Pronase content inside the capsule.
3.9. Osmotic Pressure
All the methods that use osmotic pressure as a decapsulation force are based on the same main principle of placing hydrogel from biodegradable material (such as dextran and its derivatives) into the capsule. In water, under the influence of osmotic forces, water molecules start to enter the capsule. This leads to an increase in osmotic pressure on the shell of the capsule, which, in the very end, results in shell damage facilitated by the simultaneous gradual hydrolysis of dextran. The control of decapsulation rate could be performed by changing the extent of crosslinking in the polyelectrolyte layers, the number of polyelectrolyte layers and the amount of the hydrogel inside the capsule [38,39].
3.10. Bacterially Driven Decapsulation
This type of decapsulation is based on the germination of encapsulated bacterial spores. The efficiency of this method was demonstrated in the work of Musin et al. [12]. The authors prepared microcapsules consisting of PSS/PAА that contained Bacillus subtilis spores together with FITC-dextran (Figure 2).
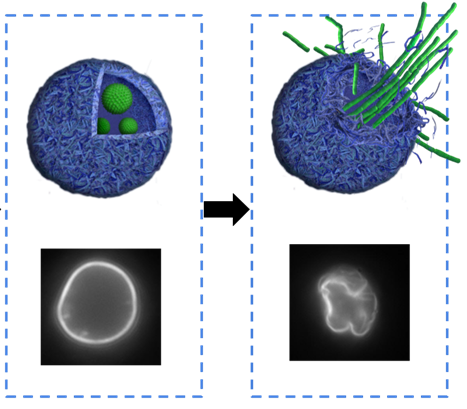
Figure 2. Scheme of bacterially driven decapsulation. (A)—PMC with encapsulated bacterial spores of Bacillus subtilis; (B)—release of FITC-dextran and destruction of PMC after spore germination.
When favorable conditions for bacterial growth were created, spores within the capsules became activated, since the created nanoscale shell was semi-permeable and allowed for nutrients to get into the capsule. When encapsulated bacterial spores germinated inside the microcapsule, they disturbed and destroyed the shell of the PMC, which resulted in decapsulation and release of the FITC-dextran. The main advantage of this decapsulation method was that it did not require any specialized, highly technological equipment, such as ultrasound or laser generators.
4. Practical Relevance and Applications
One of the main applications of microencapsulated substances is their triggered controlled release. The diversity of polyelectrolytes and nanoparticles used to create PMCs allows the development of a whole set of microcapsule types with various release triggers. Polyelectrolyte microcapsule decapsulation can be used in medicine, in genome editing, in the development of targeted delivery techniques, in agricultural and paint manufacturing industries and in ‘smart’/functional surface development.
In medicine polyelectrolyte microcapsule are mostly used for targeted drug delivery techniques [55]. The main advantage of polyelectrolyte microcapsules as drug carriers here is that the capsules protect the drug from degradation along the way to the targeted region (cell, inflammation area, disease ‘hot’ spot) and they also protect the organism from the possible side effects of the drug. One important feature for successful targeted drug delivery is its controlled release from the microcapsule without any loss of therapeutic effect [56]. Versatility of LbL technology allows the production of polymeric capsules consisting of biodegradable materials with different sizes and unique surface properties, which are required for the development of successful decapsulation strategies. Another feature of PMCs, which makes them convenient to use in targeted drug delivery, is their active engulfment by several cell types, including breast cancer cells, hepatocytes, fibroblasts, colon carcinoma cells and the model kidney cell line ‘Vero’, as well as phagocytic, dendritic cells and macrophages. In addition to this, polyelectrolyte microcapsules can be used for genetic construct delivery in genome editing techniques [57].
In the paint manufacturing industry, PMCs are used for the development of self-healing coatings. Mikhail L. Zheludkevich and coauthors created anticorrosion coatings with a self-healing effect, which consisted of hybrid sol–gel films doped with special nanocontainers. These nanocontainers were covered with the polyelectrolyte and corrosion inhibitor (benzotriazole) layers, which were released in response to pH changes caused by the corrosion process [58]. During the anticorrosion system development, pH-sensitive components, which allowed the decapsulation corrosion inhibitors, were usually used, such as ZnO [59], SiO2 nanoparticles [60], halloysite nanotubes [61,62], TiO2 nanotubes [63] and others.
Another possible application of polyelectrolyte microcapsules in the agricultural industry is the controlled release of pesticides. Such a technology would allow a decrease in the rate of active compound release and prolong its action. Moreover, it may become a solution to the problem of pesticide pollution of the environment. Xiaojing Wang and Jing showed in their work the ability to encapsulate the herbicide picloram and to perform its gradual controlled decapsulation [64].
The list of abbreviations
|
PAD |
Poly 3-acrylaminophenylboronic acid: dimethyl aminoethyl acrylate |
|
PAH-BOH |
Amide bonds between the carboxylic groups of acrilic acid and aminophenylboronic acid hemisulfate |
|
PSS |
Poly(sodium 4-styrenesulfonate) |
|
DDAB |
Dioctadecyldimethylammonium bromide |
|
MF |
Melamine formaldehyde |
|
PAH-BP |
Benzophenone-modified PAH |
|
Bt |
Benzotriazole |
|
PDADMAC |
Poly(diallyldimethylammonium chloride) |
|
PMA |
Poly(methacrylic acid) |
|
NRs |
gold nanorods |
|
DS |
Dextran sulfate |
|
PVPON |
Poly(vinylpyrrolidone) |
|
PMASH |
Poly(methacrylic acid) (PMA) conjugated with cysteamine (NH2-(CH2)2)-SH) |
|
PLL |
Poly(L-lysine) |
|
PGA |
Poly(L-glutamic acid) |
|
PSS-Rh |
Rhodamine B-labelled PSS |
|
p(HPMA-DMAE |
Poly(hydroxypropylmethacrylamide dimethylaminoethyl) |
|
CT |
Chitosan |
|
ALG |
Alginate |
|
(dex-HEMA-DMAEMA) |
Microgel dextran-hydroxyethylmethacrylate dimethylaminoethyl methacrylate |
