Alzheimer’s disease (AD) is the most common neurodegenerative disease, intensifying impairments in cognition, behavior, and memory. Histopathological AD variations include extracellular senile plaques’ formation, tangling of intracellular neurofibrils, and synaptic and neuronal loss in the brain. Multiple evidence directly indicates that oxidative stress participates in an early phase of AD before cytopathology. Oxidative stress plays a crucial role in activating and causing various cell signaling pathways that result in lesion formations of toxic substances, which advances the disease. Antioxidants are widely preferred to combat oxidative stress, and those derived from natural sources, which are often incorporated into dietary habits, can play an important role in delaying the onset as well as reducing the progression of AD. However, this approach has not been extensively explored yet. Moreover, a combination of antioxidants in conjugation with a nutrient-rich diet might be more effective in tackling AD pathogenesis.
- Alzheimer’s disease
- antioxidants
- oxidative stress
- reactive oxygen species
- therapeutics
1. Role of Antioxidant-Rich Diet in Alzheimer’s Disease
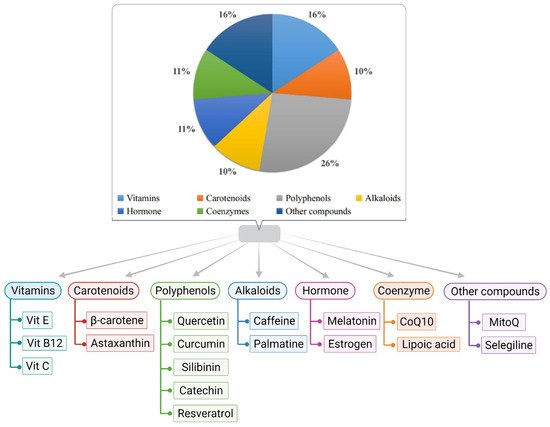
2. Role of Antioxidants in Alzheimer’s Disease
2.1. Vitamin E
2.2. Glutathione
2.3. Molecular Hydrogen
2.4. Monoamine Oxidase-b Inhibitor
2.5. Melatonin
2.6. Ascorbyl Palmitate
2.7. Curcumin
2.8. Coenzyme Q and SK-PC-B70M
2.9. Estrogen, Astaxanthin, and Quercetin
2.10. Lipoic Acid
2.11. Resveratrol
2.12. MitoQ
2.13. Catechins
2.14. Silibinin
2.15. Palmatine
2.16. Serotonin
2.17. Gintonin
3. Role of Other Nutrients in Alzheimer’s Disease
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biology11020212
