Heme oxygenase 1 (HO-1), the rate-limiting enzyme in heme degradation, is involved in the maintenance of cellular homeostasis, exerting a cytoprotective role by its antioxidative and anti-inflammatory functions. HO-1 and its end products, biliverdin (BV), carbon monoxide (CO) and free iron (Fe2+), confer cytoprotection against inflammatory and oxidative injury. Additionally, HO-1 exerts antiviral properties against a diverse range of viral infections by interfering with replication or activating the interferon (IFN) pathway. Severe cases of coronavirus disease 2019 (COVID-19), an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), are characterized by systemic hyperinflammation, which, in some cases, leads to severe or fatal symptoms as a consequence of respiratory failure, lung and heart damage, kidney failure, and nervous system complications. Here we summarize the current research on the protective role of HO-1 in inflammatory diseases and against a wide range of viral infections, positioning HO-1 as an attractive target to ameliorate clinical manifestations during COVID-19.
- heme oxygenase 1
- COVID-19
- influenza A virus
- respiratory syncytial virus
- human immunodeficiency virus
- Ebola virus
- Dengue virus
- Zika virus
- SARS-CoV-2
1. Introduction
The treatment goal in COVID-19 patients is to prevent or to decrease the strong virus induced inflammatory stimuli associated with a wide spectrum of poor prognosis clinical manifestations [1]. HO-1 is a microsomal enzyme with a primary antioxidant and anti-inflammatory role involved in heme degradation, generating CO, BV, and Fe2+ [2]. Hence, HO-1 induction is a useful approach for inflammatory diseases treatment [3][4][5][6]. Additionally, HO-1 displays antiviral properties against a wide range of viruses [7]. Hemin, a previously Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved drug for acute intermittent porphyria treatment [8][9], is a well known inducer of HO-1 that increases its plasma concentration in humans. Thus, hemin rises as a promising drug candidate against the replication of different viruses, including SARS-CoV-2.
2. Cytokine Storm and Inflammation
3. The Lead Role of Interferons upon Viral Infection
4. Understanding the Protective Role of Heme Oxygenase 1
5. HO-1 Mechanism of Action against Inflammatory Lung Diseases
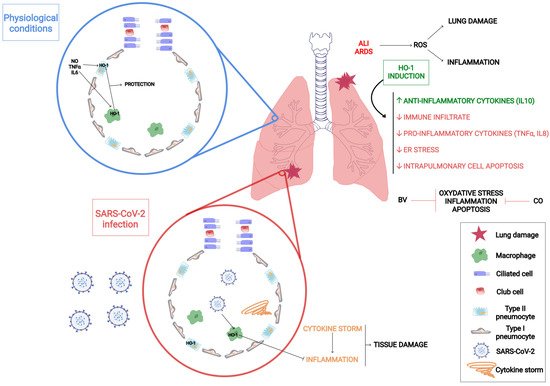
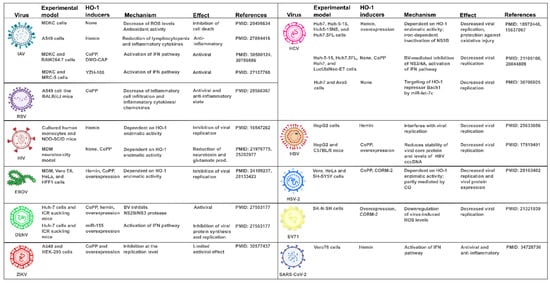
6. Unveiling How HO-1 Promotes Viral Clearance
7. HO-1 Induction as a Strategy against COVID-19
9. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/antiox11020276
References
- Marcella Prete; Elvira Favoino; Giacomo Catacchio; Vito Racanelli; Federico Perosa; SARS-CoV-2 Inflammatory Syndrome. Clinical Features and Rationale for Immunological Treatment. International Journal of Molecular Sciences 2020, 21, 3377, 10.3390/ijms21093377.
- Louise L. Dunn; Robyn G. Midwinter; Jun Ni; Hafizah A. Hamid; Christopher Parish; Roland Stocker; New Insights into Intracellular Locations and Functions of Heme Oxygenase-1. Antioxidants & Redox Signaling 2014, 20, 1723-1742, 10.1089/ars.2013.5675.
- Y. Naito; T. Takagi; T. Yoshikawa; Heme oxygenase-1: a new therapeutic target for inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 2004, 20, 177-184, 10.1111/j.1365-2036.2004.01992.x.
- Tomohisa Takagi; Yuji Naito; Katsura Mizushima; Yasuko Hirai; Akihito Harusato; Tetsuya Okayama; Kazhuhiro Katada; Kazuhiko Uchiyama; Osamu Handa; Takeshi Ishikawa; et al. Heme oxygenase-1 prevents murine intestinal inflammation. Journal of Clinical Biochemistry and Nutrition 2017, 63, 169-174, 10.3164/jcbn.17-133.
- Ângelo Ferreira Chora; Paulo Fontoura; Andreia Cunha; Teresa Faria Pais; Sílvia Cardoso; Peggy P. Ho; Lowen Y. Lee; Raymond A. Sobel; Lawrence Steinman; Miguel P. Soares; et al. Heme oxygenase–1 and carbon monoxide suppress autoimmune neuroinflammation. Journal of Clinical Investigation 2007, 117, 438-447, 10.1172/jci28844.
- Ulrike Protzer; Stefan Seyfried; Maria Quasdorff; Gabriele Sass; Miriam Svorcova; Dennis Webb; Felix Bohne; Marianna Hösel; Peter Schirmacher; Gisa Tiegs; et al. Antiviral Activity and Hepatoprotection by Heme Oxygenase-1 in Hepatitis B Virus Infection. Gastroenterology 2007, 133, 1156-1165, 10.1053/j.gastro.2007.07.021.
- Janyra A. Espinoza; Pablo A. González; Alexis M. Kalergis; Modulation of Antiviral Immunity by Heme Oxygenase-1. The American Journal of Pathology 2017, 187, 487-493, 10.1016/j.ajpath.2016.11.011.
- Karl E. Anderson; Stephen Collins; Open-Label Study of Hemin for Acute Porphyria: Clinical Practice Implications. The American Journal of Medicine 2006, 119, 801.e1-801.e6, 10.1016/j.amjmed.2006.05.026.
- List of nationally authorised medicinal products . European Medicines Agency. Retrieved 2022-2-9
- Riccardo V. D'elia; Kate Harrison; Petra C. Oyston; Roman A. Lukaszewski; Graeme C. Clark; Targeting the “Cytokine Storm” for Therapeutic Benefit. Clinical and Vaccine Immunology 2013, 20, 319-327, 10.1128/cvi.00636-12.
- Linlin Chen; Huidan Deng; Hengmin Cui; Jing Fang; Zhicai Zuo; Junliang Deng; Yinglun Li; Xun Wang; Ling Zhao; Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204-7218, 10.18632/oncotarget.23208.
- Ruslan Medzhitov; Recognition of microorganisms and activation of the immune response. Nature Cell Biology 2007, 449, 819-826, 10.1038/nature06246.
- Cecilia L. Speyer; Peter A. Ward; Role of Endothelial Chemokines and Their Receptors during Inflammation. Journal of Investigative Surgery 2011, 24, 18-27, 10.3109/08941939.2010.521232.
- Lan Yang; Xueru Xie; Zikun Tu; Jinrong Fu; Damo Xu; Yufeng Zhou; The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduction and Targeted Therapy 2021, 6, 1-20, 10.1038/s41392-021-00679-0.
- Matthew Zirui Tay; Chek Meng Poh; Laurent Rénia; Paul A. Macary; Lisa F. P. Ng; The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology 2020, 20, 363-374, 10.1038/s41577-020-0311-8.
- Carolina Lucas; Patrick Wong; Jon Klein; Tiago B. R. Castro; Julio Silva; Maria Sundaram; Mallory K. Ellingson; Tianyang Mao; Ji Eun Oh; Benjamin Israelow; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463-469, 10.1038/s41586-020-2588-y.
- Daniel Blanco-Melo; Benjamin E. Nilsson-Payant; Wen-Chun Liu; Skyler Uhl; Daisy Hoagland; Rasmus Møller; Tristan X. Jordan; Kohei Oishi; Maryline Panis; David Sachs; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036-1045.e9, 10.1016/j.cell.2020.04.026.
- Annsea Park; Akiko Iwasaki; Type I and Type III Interferons – Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host & Microbe 2020, 27, 870-878, 10.1016/j.chom.2020.05.008.
- Sandra Hervas-Stubbs; Jose Luis Perez-Gracia; Ana Rouzaut; Miguel F Sanmamed; Agnes Le Bon; Ignacio Melero; Direct Effects of Type I Interferons on Cells of the Immune System. Clinical Cancer Research 2011, 17, 2619-2627, 10.1158/1078-0432.ccr-10-1114.
- David E. Levy; The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine & Growth Factor Reviews 2001, 12, 143-156, 10.1016/s1359-6101(00)00027-7.
- S. Goodbourn; L. Didcock; R. E. Randall; Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. Journal of General Virology 2000, 81, 2341-2364, 10.1099/0022-1317-81-10-2341.
- Colleen Cebulla; Daniel M. Miller; Daniel D. Sedmak; Viral Inhibition of Interferon Signal Transduction. Intervirology 1998, 42, 325-330, 10.1159/000053968.
- Sanjay Kumar; Uday Bandyopadhyay; Free heme toxicity and its detoxification systems in human. Toxicology Letters 2005, 157, 175-188, 10.1016/j.toxlet.2005.03.004.
- Shunsuke Hayashi; Yoshiaki Omata; Hiroshi Sakamoto; Yuichiro Higashimoto; Takayuki Hara; Yasuhiro Sagara; Masato Noguchi; Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 2004, 336, 241-250, 10.1016/j.gene.2004.04.002.
- Akihiro Yachie; Yo Niida; Taizo Wada; Noboru Igarashi; Hisashi Kaneda; Tomoko Toma; Kazuhide Ohta; Yoshihito Kasahara; Shoichi Koizumi; Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. Journal of Clinical Investigation 1998, 103, 129-135, 10.1172/jci4165.
- Yunlong Li; Kuai Ma; Zhongyu Han; Mingxuan Chi; Xiyalatu Sai; Ping Zhu; Zhaolun Ding; Linjiang Song; Chi Liu; Immunomodulatory Effects of Heme Oxygenase-1 in Kidney Disease. Frontiers in Medicine 2021, 8, -, 10.3389/fmed.2021.708453.
- Kun-Chun Chiang; Kang-Shuo Chang; Shu-Yuan Hsu; Hsin-Ching Sung; Tsui-Hsia Feng; Mei Chao; Horng-Heng Juang; Human Heme Oxygenase-1 Induced by Interleukin-6 via JAK/STAT3 Pathways Is a Tumor Suppressor Gene in Hepatoma Cells. Antioxidants 2020, 9, 251, 10.3390/antiox9030251.
- Geraldine Gueron; Adriana De Siervi; Mercedes Ferrando; Marcelo Salierno; Paola De Luca; Belen Elguero; Roberto Meiss; Nora Navone; Elba Vazquez; Critical Role of Endogenous Heme Oxygenase 1 as a Tuner of the Invasive Potential of Prostate Cancer Cells. Molecular Cancer Research 2009, 7, 1745-1755, 10.1158/1541-7786.mcr-08-0325.
- S Immenschuh; H Schröder; Heme oxygenase-1 and cardiovascular disease. Histol. Histopathol. 2006, 21, 679-685, 10.14670/HH-21.679.
- Xiaoliang Lin; Jiajia Lv; Dandan Ge; Haitao Bai; Yungang Yang; Jinzhun Wu; Heme oxygenase‐1 alleviates eosinophilic inflammation by inhibiting STAT3‐SOCS3 signaling. Pediatric Pulmonology 2020, 55, 1440-1447, 10.1002/ppul.24759.
- Shehzad Z. Sheikh; Refaat A. Hegazi; Taku Kobayashi; Joseph C. Onyiah; Steven M. Russo; Katsuyoshi Matsuoka; Antonia R. Sepulveda; Fengling Li; Leo E. Otterbein; Scott E. Plevy; et al. An Anti-Inflammatory Role for Carbon Monoxide and Heme Oxygenase-1 in Chronic Th2-Mediated Murine Colitis. The Journal of Immunology 2011, 186, 5506-5513, 10.4049/jimmunol.1002433.
- Ananta Paine; Britta Eiz-Vesper; Rainer Blasczyk; Stephan Immenschuh; Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology 2010, 80, 1895-1903, 10.1016/j.bcp.2010.07.014.
- Sotiria Tzima; Panayiotis Victoratos; Ksanthi Kranidioti; Maria Alexiou; George Kollias; Myeloid heme oxygenase–1 regulates innate immunity and autoimmunity by modulating IFN-β production. Journal of Experimental Medicine 2009, 206, 1167-1179, 10.1084/jem.20081582.
- Katja Kotsch; Paulo N.A. Martins; Roman Klemz; Uwe Janssen; Bernhard Gerstmayer; Annelie Dernier; Anja Reutzel-Selke; Ulrike Kuckelkorn; Stefan G. Tullius; Hans-Dieter Volk; et al. Heme Oxygenase-1 Ameliorates Ischemia/Reperfusion Injury by Targeting Dendritic Cell Maturation and Migration. Antioxidants & Redox Signaling 2007, 9, 2049-2064, 10.1089/ars.2007.1801.
- Christine Chauveau; Séverine Rémy; Pierre Joseph Royer; Marcelo Hill; Séverine Tanguy-Royer; François-Xavier Hubert; Laurent Tesson; Régis Brion; Gaëlle Beriou; Marc Grégoire; et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 2005, 106, 1694-1702, 10.1182/blood-2005-02-0494.
- Matthias H. Kapturczak; Clive Wasserfall; Todd Brusko; Martha Campbell-Thompson; Tamir M. Ellis; Mark A. Atkinson; Anupam Agarwal; Heme Oxygenase-1 Modulates Early Inflammatory Responses: Evidence from the Heme Oxygenase-1-Deficient Mouse. The American Journal of Pathology 2004, 165, 1045-1053, 10.1016/s0002-9440(10)63365-2.
- Leo E. Otterbein; Fritz H. Bach; Jawed Alam; Miguel Soares; Hong Tao Lu; Mark Allen Wysk; Roger J. Davis; Richard A. Flavell; Augustine M. K. Choi; Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature Medicine 2000, 6, 422-428, 10.1038/74680.
- Tzong-Shyuan Lee; Lee-Young Chau; Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature Medicine 2002, 8, 240-246, 10.1038/nm0302-240.
- Miguel P. Soares; Mark P. Seldon; Isabel Pombo Gregoire; Tatiana Vassilevskaia; Pascal O. Berberat; Jia Yu; Tung-Yu Tsui; Fritz H. Bach; Heme Oxygenase-1 Modulates the Expression of Adhesion Molecules Associated with Endothelial Cell Activation. The Journal of Immunology 2004, 172, 3553-3563, 10.4049/jimmunol.172.6.3553.
- Toshitaka Nakamura; Isao Naguro; Hidenori Ichijo; Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochimica et Biophysica Acta (BBA) - General Subjects 2019, 1863, 1398-1409, 10.1016/j.bbagen.2019.06.010.
- Laura E. Fredenburgh; Mark A. Perrella; S. Alex Mitsialis; The Role of Heme Oxygenase-1 in Pulmonary Disease. American Journal of Respiratory Cell and Molecular Biology 2007, 36, 158-165, 10.1165/rcmb.2006-0331tr.
- Dirk-Jan Slebos; Stefan W Ryter; Augustine M K Choi; Heme oxygenase-1 and carbon monoxide in pulmonary medicine. Respiratory Research 2003, 4, 7-7, 10.1186/1465-9921-4-7.
- Caterina Di Pietro; Hasan H. Öz; Thomas S. Murray; Emanuela M. Bruscia; Targeting the Heme Oxygenase 1/Carbon Monoxide Pathway to Resolve Lung Hyper-Inflammation and Restore a Regulated Immune Response in Cystic Fibrosis. Frontiers in Pharmacology 2020, 11, 1059, 10.3389/fphar.2020.01059.
- B. Müller; Hans-Georg Kräusslich; Antiviral Strategies. Diabetes - Perspectives in Drug Therapy 2008, 189, 1-24, 10.1007/978-3-540-79086-0_1.
- John Kenneth Baillie; Paul Digard; Influenza — Time to Target the Host?. New England Journal of Medicine 2013, 369, 191-193, 10.1056/nejmcibr1304414.
- Mack Sheraton; Neha Deo; Rahul Kashyap; Salim Surani; A Review of Neurological Complications of COVID-19. Cureus 2020, 12, e8192, 10.7759/cureus.8192.
- Rose H. Manjili; Melika Zarei; Mehran Habibi; Masoud H. Manjili; COVID-19 as an Acute Inflammatory Disease. The Journal of Immunology 2020, 205, 12-19, 10.4049/jimmunol.2000413.
- Neelu Batra; Cristabelle De Souza; Jyoti Batra; Alan Raetz; Ai-Ming Yu; The HMOX1 Pathway as a Promising Target for the Treatment and Prevention of SARS-CoV-2 of 2019 (COVID-19). International Journal of Molecular Sciences 2020, 21, 6412, 10.3390/ijms21176412.
- Yonghua Zhu; Yunjuan Sun; Kunlin Jin; David A. Greenberg; Hemin induces neuroglobin expression in neural cells. Blood 2002, 100, 2494-2498, 10.1182/blood-2002-01-0280.
- Hui‑Min Li; Ying‑Lu Shi; Di Wen; Huan‑Min Luo; Xi Lin; Fei Xiao; A novel effective chemical hemin for the treatment of acute carbon monoxide poisoning in mice. Experimental and Therapeutic Medicine 2017, 14, 5186-5192, 10.3892/etm.2017.5157.
- Xiangping Lu; Jing Chen-Roetling; Raymond F. Regan; Systemic hemin therapy attenuates blood–brain barrier disruption after intracerebral hemorrhage. Neurobiology of Disease 2014, 70, 245-251, 10.1016/j.nbd.2014.06.005.
- David Olagnier; Ensieh Farahani; Jacob Thyrsted; Julia Blay-Cadanet; Angela Herengt; Manja Idorn; Alon Hait; Bruno Hernaez; Alice Knudsen; Marie Beck Iversen; et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nature Communications 2020, 11, 1-12, 10.1038/s41467-020-18764-3.
- Antonio Cuadrado; Marta Pajares; Cristina Benito; José Jiménez-Villegas; Maribel Escoll; Raquel Fernández-Ginés; Angel J. Garcia Yagüe; Diego Lastra; Gina Manda; Ana I. Rojo; et al. Can Activation of NRF2 Be a Strategy against COVID-19?. Trends in Pharmacological Sciences 2020, 41, 598-610, 10.1016/j.tips.2020.07.003.
