Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
Drosophila melanogaster has provided a new dimension to the discipline of neuroscience. The research on olfactory and gustatory systems using fruit flies has grown rapidly during the last few years due to the strong genetic similarity that humans share with them.
- Drosophila melanogaster
- gustation
- olfaction
- gustatory receptors
- olfactory receptors
- insect repellents
- ionotropic receptors
1. Introduction
This has proved instrumental in establishing D. melanogaster as an ideal model organism. Additionally, fruit flies offer additional advantages to researchers over humans or mammals. The flies can be easily cultured in laboratory conditions and are quite inexpensive to maintain. They produce a large number of eggs and have a relatively short life cycle that provides much-needed flexibility to the researchers. The flies can also be genetically modified [1] and the neurons easily detected in every individual fly [2]. The larval stages of the fly can be used for different experiments, since they are simple in structural organization and easy to handle as compared to adult flies.
Olfaction plays a vital role in the survival of D. melanogaster. The process commences with the binding of volatile odorant molecules to their specific receptors known as odorant receptors (Ors), which are expressed on the olfactory sensory neurons (OSNs). In the larval stages, each of the two dorsal organs (DO) have 21 OSNs [3,4], which contrarily, are contained in specialized sensory hairs known as sensilla, situated on the third antennal segments and maxillary palps in the adult flies. Earlier studies on olfaction in D. melanogaster used odorants in association with an appetitive or an aversive stimulus. The naïve D. melanogaster larvae showed vigorous chemotaxis towards many odorants, including ethyl acetate (EA) [5]. It was observed that the behavioral responses of larvae were diametrically opposite to shorter and longer chain acetates. The former (methyl to pentyl acetates) were attractive in nature, whereas the latter (hexyl to octyl acetates) triggered repulsion [6]. Besides this, the flies effectively avoided odorants which were paired with an electric shock in learning and memory experiments [7]. An age-dependent decline in olfactory response/memory was reported in D. melanogaster [8] and in those offspring born to aging flies [9]. These behavioral assays based on odorants have played a major role in discovering specific genes involved in the olfactory pathways [10,11] and the regions of the insect brain controlling them.
In gustation, there is a remarkable similarity of food choices and their detection that fruit flies share with mammals. This has given opportunities to researchers to conduct in-depth studies of taste perception. Both mammals and fruit flies consume carbohydrates as their major food source, and both avoid chemicals that are either toxic or taste bitter. The sensitivity and range of recognition of gustatory receptors (Grs) of D. melanogaster are similar to mammals. The Grs are mainly localized in the head and pharyngeal regions of the larvae [12], while in adult flies, they are dispersed on the mouthparts, leg tarsi, and around the female ovipositor. The wings of D. melanogaster also possess taste sensilla. The margins of the anterior wings respond to both appetitive and aversive stimuli due to increases in Ca2+ ions in the cytoplasm [13]. The Grs detecting food items are well characterized in D. melanogaster with a single Gr responding to fructose [14], but the case is different in bitter taste receptors. Several researchers have proposed bitter taste Grs to exhibit a multimeric organization comprising of one subunit tuned to a limited specificity, while others function as broadly required co-receptors [15,16,17,18,19,20]. The role of the intestinal gut of fruit flies in perceiving gustatory stimuli has also been reviewed. Due to the presence of Gr transcripts, the intestinal gut controls many biological functions such as ingestion, absorption of nutrients, and balancing body sugar levels [21].
The in-depth understanding of the chemosensory machinery of D. melanogaster has great practical applications, which may be implemented to design new methods of controlling disease-causing insect vectors and crop pests, and to gain a better understanding of how the batches of insect and pest repellents that are currently available in the market function. Insects are one of the major sources of the transmission of pathogens to humans and cattle, as well as the destruction of food crops [22]. Mosquitoes, ticks, sandflies, and mites are a few of the major insect vectors that spread life-threatening diseases to human beings, such as; malaria, dengue, West Nile fever, encephalitis, yellow fever, and Congo hemorrhagic disease [23]. In particular, mosquitoes are the biggest menace to the human population. Anopheles mosquitoes, i.e., An. Gambiae and An. Funestus, are involved in the transmission of the malarial parasite, Plasmodium sp., to their human hosts, triggering one million annual deaths [24,25]. The human filarial nematode Wuchereria bancrofti, and arboviruses spread through the bites of Culex mosquitoes [25] and Aedes aegypti (the yellow fever mosquito), account for many cases of dengue globally [25,26]. As regards crop pests, the facts are disturbing; for example, the spotted wing Drosophila suzukii, an invasive global insect pest, causes huge crop loss by destroying fresh, ripened small fruits and tree fruits at large scale [27,28,29,30].
2. Olfactory System—Components and Basic Organization
In D. melanogaster larvae, the olfactory system is mainly localized to the head region. The head portion of larvae has a couple of DOs expressing 21 OSNs in each [3,4] (Figure 1A). Contrarily, the adult fruit flies detect odorants through a pair of antennae and maxillary palps (Figure 1B). These appendages are positioned on the head region, enveloped with numerous sensory hairs called sensilla. The sensilla possess OSNs that are specialized in detecting odorants (Figure 2A). Each antenna has about 410 olfactory sensilla with nearly 1300 OSNs, whereas the number of olfactory sensilla is up to 60 in each maxillary palp with approximately 120 OSNs [31,32,33,34]. These sensory hairs exhibit differences in their morphology. A recent work on serial block-face scanning electron microscopy (SBEM) images of antennal tissues has further led to a systematic morphological and morphometric analysis of the identified olfactory sensilla in D. melanogaster. A plethora of new information, such as inner dendritic enlargement with enhanced mitochondrial content, the presence of extracellular vacuoles in the lumen of sensilla, empty sensilla with no OSNs, and two new unconventional types of basiconic sensory hairs, was discovered. The olfactory sensilla in fruit flies can be separated into four different groups [35] (Figure 2B), which are as follows:
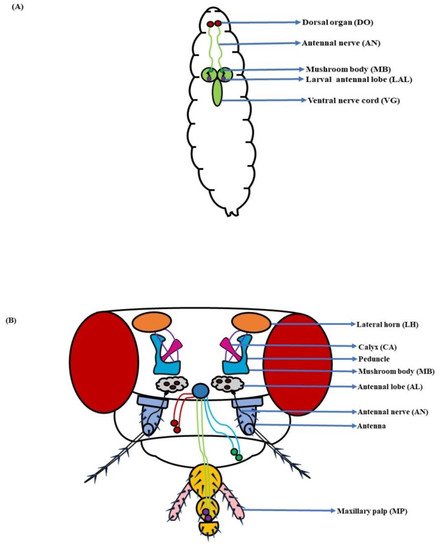
Figure 1. Components of the olfactory system: (A) Larva: With a much smaller and simplified architecture, the dorsal organ (DO), antennal nerve (AN), mushroom body (MB), larval antennal lobe (LAL), and ventral nerve cord (VG) constitute the larval olfactory system; (B) Adult: The antenna and maxillary palp (MP) along with the antennal lobe (AL), antennal nerve (AN), mushroom body (MB), and lateral horn (LH), complete the fly’s olfactory system (Modified from [36,37,38,39]).
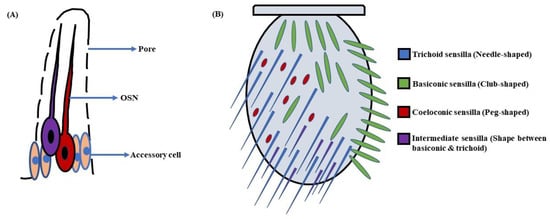
Figure 2. Schematic of (A) Sensillum: A sensillum consists of accessory cells, olfactory sensory neurons (OSNs), and pores for odorant molecules to enter; (B) Antenna: The antennae in D. melanogaster’s head are covered with sensory hairs called sensilla. These are of four types, namely trichoid (needle-shaped), basiconic (club-shaped), coeloconic (peg-shaped), and intermediate (shape between basiconic & trichoid) sensilla (Modified from [40]).
a. Basiconic sensilla: The sensory hairs at the proximomedial region of the antenna are club-shaped and are called basiconic sensilla. These sensilla are of 10 types, viz. ab1-ab10, are further grouped into three sub-classes, i.e., large, small, and thin. The large basiconic sensilla (ab1–ab3), ~12 µm in length, house two or four OSNs, whereas small basiconic sensilla (ab7–ab10), which are ~9 µm long, enclose two OSNs. The thin basiconic sensilla (ab4–ab6) are also ~12 µm in length but are thinner in shape. They encompass mostly two with a few cases of four OSNs per sensillum [31,41]. Additionally, there are also two novel types of basiconic sensilla; one is a large basiconic sensillum, abx(3), which encloses three OSNs, and the other is a small basiconic sensillum, abx(1), with a single OSN. Interestingly, one small basiconic sensillum with no OSN is also known to exist, which is designated as abx(0) [35]. The sensory hairs on the maxillary palp are all thin basiconic (pb1–pb3), each housing two OSNs [41]. The basiconic sensilla are single-walled.
b. Trichoid sensilla: The trichoid sensilla are pointed in shape and vary in length from 18–22 µm [31]. They occupy the lateral profile of the antenna, predominantly at the distal tip. These sensilla are of four types, namely at1-at4, and are classified as T1 (at1), T2 (at2), and T3 (at3 & at4) corresponding to the number of OSNs they house, i.e., one, two, and three, respectively [31,41]. However, Fluorescence-guided Single Sensillum Recording (FgSSR) analyses have re-categorized two trichoid sensilla, namely at2 and at3, as intermediate sensilla ai2 and ai3 [42]. Besides this, one trichoid sensillum belonging to the T1 subtype with no OSN has also been found in fruit flies and named T(0) [35]. The trichoid sensilla are also single-walled.
c. Intermediate sensilla: There are a few sensory hairs in the antenna exhibiting length and structure in between trichoid and basiconic sensilla. These are called intermediate sensilla. They are scattered among the trichoids on the frontal antennal surface, with their number varying from 10–20 on each antenna [31,41]. The intermediate sensilla are of two types, namely ai2 and ai3, housing two and three OSNs respectively [42]. They are single-walled.
d. Coeloconic sensilla: The coeloconic sensilla are the smallest of all the sensory hairs. They are short in size (~5 µm) and peg-shaped. Although they are dispersed with other groups of sensilla, the majority of them cover the posterior surface of the antenna. The number of OSNs in coeloconic sensilla varies between two to four neurons per coeloconic sensillum [31,41,43]. The ac3 sensillum houses two OSNs, ac2 and ac4, housing three OSNs each, whereas ac1 compartmentalizes four OSNs [35]. These sensory hairs are double-walled.
Further, in D. melanogaster, the antennal lobe (AL) functions as a deutocerebral neuropil showing functional similarities to the olfactory bulb of the vertebrate brain [44]. At larval stage, the antennal nerve (AN) connects 21 OSNs in DO to the larval antennal lobe (LAL). The LAL has about 30 subunits comparable to the glomerulus of an adult fly [4,45]. The sensory information from LAL then goes through several projections and local neurons (PNs & LNs) to be relayed to higher centers of the larval brain to initiate a behavioral response (Figure 3A). Similarly, in adult flies, OSNs located in the antenna relay sensory information to the AL, consisting of tightly packed neuropils called glomeruli. The number of glomeruli in D. melanogaster is 54 per AL, out of which 52 are innervated by chemosensory and 2 by thermosensory neurons [46,47]. At the glomeruli, the electric signals are further relayed to two specific classes of neurons, the PNs and LNs. The PNs housed in the AL project their axons to the protocerebrum [41] and from here on to the mushroom body (MB) and lateral horn (LH). Thus, the information from the PNs is transferred to these paired neural organs, which comprise higher centers of the fly’s brain [48,49], (Figure 3B).
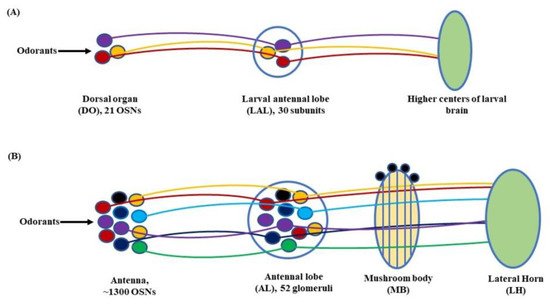
Figure 3. Olfactory signaling pathway of (A) Larva: At the larval stage, the odorant molecules are sensed by the dorsal organ (DO). The stimulus is then transferred to the larval antennal lobe (LAL) by the olfactory sensory neurons (OSNs) housed in the DO. From here onwards, through a grid of projection neurons (PNs) and local neurons (LNs) the electrical signal is projected to the larval brain to elicit a response; (B) Adult: In fruit flies, the olfactory signaling begins with the antennae and maxillary palps. The OSNs, with the help of odorant receptors (Ors), carry the olfactory cue to the antennal lobe (AL). From there on, through a network of PNs and LNs, the stimulus is transferred to the mushroom body (MB) and lateral horn (LH) to initiate a response (Modified from [34,50]).
2.1. Olfactory Sensory Neurons (OSNs)
The OSNs form the most important aspect of olfactory detection in D. melanogaster. Their numbers exhibit great variations between the larval and adult stages of the fly. In larvae there are 21 OSNs per DO, whereas the adult fruit flies house much greater number of neurons. Each antenna displays ~1300 OSNs, whereas the actual number is 120 in each maxillary palp. An individual OSN typically embodies a single Or, although a few multiple receptor OSNs are also present in D. melanogaster [44,51]. The OSNs expressing identical receptor gene/genes then project to a specific glomerulus [52,53], where they synapse onto PNs and LNs [54] for further processing of the olfactory cues. A few studies have suggested that the number of OSNs converging into glomeruli varies between 52–53 OSNs per glomerulus [55]. However, each glomeruli has its own unique neuronal composition and glomerular volume, which is a result of varying numbers of OSNs and uniglomerular PNs received by different sets of glomeruli [56]. Additionally, an increased number of glomeruli per antennal lobe has been reported in D. melanogaster. The number is now 58, out of which 51 are olfactory [57] and 7, i.e., VP1d, VP1l, VP1m [58], VP2, VP3 [59], and VP4 [60,61,62] are thermo/hygrosensory in nature. They house a total of ~2600 antennal lobe sensory neurons (ALSNs) including OSNs and thermo/hygrosensory neurons (T/HSNs) [63].
2.2. Odorant Receptors (Ors)
The odorant receptors (Ors) in D. melanogaster are membrane-associated proteins by nature. They are encoded and expressed by Or genes [64,65]. The seven transmembrane domains possessed by these proteins are quite distinct and have no homology to the vertebrate GPCRs or Ors [66,67,68]. There are 60 Or genes in D. melanogaster, which encode 62 Ors by the process of alternative splicing (Table 1) [69,70,71]. However, studies using transgenic reporter techniques have changed the scenario to a considerable effect. These studies have revealed that the count of genes exclusive for antennal Ors is 40, whereas 7 genes translate receptors specific to maxillary palps [34,41,52]. The remaining Or genes are involved in the encoding of larval Ors and are not detectably expressed in adult fruit flies. There are 25 larval Ors, of which 13 are specifically expressed in larvae [70,72,73]. Additionally, Ors exhibit a unique feature. The receptors expressed in the antennae and maxillary palps of D. melanogaster are exclusive to them and are not expressed in each other, as established by several algorithmic studies. These studies have identified a dyad element that promotes the expression of maxillary-palp-specific Ors and a motif that represses their expression in the antennae [74]. One more restrictive feature of Ors is that the expression of Or genes in morphologically distinct sensilla follows a set pattern [75]. The study of this pattern has revealed a strong correlation between the developmental pathways of the sensilla and the types of Ors they express [32].
Table 1. Odorant receptors (Ors) in Drosophila melanogaster.
| Olfactory Sensory Neuron | Odorant Receptor | Glomerulus |
|---|---|---|
| - | Or1a * | - |
| ai3 | Or2a | DA4m |
| ab4A | Or7a | DL5 |
| ab8B | Or9a | VM3 |
| ab1D | Or10a | DL1 |
| ab6A | Or13a | DC2 |
| ai3A | Or19a | DC1 |
| ai3A | Or19b | DC1 |
| ab3A | Or22a | DM2 |
| ab3A | Or22b | DM2 |
| - | Or22c * | - |
| ai2B | Or23a | DA3 |
| - | Or24a * | - |
| - | Or30a * | - |
| ab4B | Or33a | DA2 |
| ab2B, ab5B | Or33b | DM5, DM3 |
| pb2A | Or33c | VC1 |
| ac3B | Or35a | VC3 |
| pb1A | Or42a | VM7d |
| ab1A | Or42b | DM1 |
| ai3 | Or43a | DA4l |
| ab8A | Or43b | VM2 |
| - | Or45a * | - |
| - | Or45b * | - |
| pb2B | Or46a | VA71 |
| ab5B | Or47a | DM3 |
| at4A | Or47b | VA1v |
| ab10B | Or49a | DL4 |
| ab6B | Or49b | VA5 |
| ab4B | Or56a | DA2 |
| - | Or59a * | - |
| ab2A | Or59b | DM4 |
| pb3A | Or59c | VM7v |
| - | Or63a * | - |
| at4B | Or65a | DL3 |
| at4B | Or65b | DL3 |
| at4B | Or65c | DL3 |
| ab10A | Or67a | DM6 |
| ab9 | Or67b | VA3 |
| ab7B | Or67c | VC4 |
| at1A | Or67d | DA1 |
| ab9 | Or69a | D |
| pb1B | Or71a | VC2 |
| - | Or74a * | - |
| ab5A | Or82a | VA6 |
| - | Or83a * | - |
| ab, ai, at, pb, ac3 | Or83b/Orco | DA, DC, DL, DM, VA, VC, VM |
| ai2A | Or83c | DC3 |
| ab2B | Or85a | DM5 |
| ab3B | Or85b | VM5d |
| - | Or85c * | - |
| pb3B | Or85d | VA4 |
| pb2A | Or85e | VC1 |
| ab10B | Or85f | DL4 |
| at4C | Or88a | VA1d |
| ab1B | Or92a | VA2 |
| - | Or94a * | - |
| - | Or94b * | - |
| ab7A | Or98a | VM5v |
| ab6 | Or98b | VM5d |
Outline of odorant receptors (Ors), their OSN classes, and targeted glomeruli. Larval specific Ors are marked with asterisk *. (Adapted from [76,77]).
It is well-established that a solo Or gene is translated in each OSN [41,78,79]. However, a few studies have revealed interesting facts about Or gene expression. Each D. melanogaster OSN expressing an Or also expresses a co-receptor named as Orco/Or83b [65,80]. This co-receptor is essential to the appropriate ciliary routing and functioning of every single Or [65,81]. The flies devoid of Orco exhibit defective behavioral and electrophysiological responses to a number of odorants [67]. Besides this, four populations of OSNs co-express two conventional Ors (Or33a/Or56a, Or33b/Or47a, Or33b/Or85a and Or33c/Or85e) and a fifth one co-expresses one Or and one Gr (Or10a/Gr10a) along with the co-receptor Orco leading to a modulated ligand response profile [51,52]. A couple of recent works using cryo-electron microscopy have further given an insight into the functioning of Or/Orco heteromeric complexes. Butterwick et al., through structural analyses, has found that an Orco homomer consists of four subunits organized in a symmetrical fashion around a central pore. Each Orco subunit is further composed of seven transmembrane helical segments (S1–S7), out of which S7a (the cytoplasmic section) forms the crux of the anchor domain, whereas S7b (the transmembrane section) contours the central pore. The pore is narrowest at the extracellular end of S7b due to the presence of hydrophobic residues Leu473 and Val469. When a ligand binds to the Or/Orco heteromeric complex, the hydrophobic aperture is expanded. This central ion-conduction pathway then veers into the four lateral channels (6-Å long) that open to the cytosol, providing an uninterrupted pathway for the transfer of ions [82]. On a similar note, Marmol et al. has deciphered that the odorant receptor MhOr5 in Machilis hrabei exhibits identical quadrivial structural organization and functions similarly to Orco. Here, the binding of the ligand also dilates the S7b helices to gate the ion conduction pathway. In nutshell, these structural understandings of the Or and Orco have shed a light on the promiscuous nature of these receptors, allowing insects to have a versatile chemical recognition system [83].
2.3. Ionotropic Receptors (Irs)
Besides Ors, an additional group of receptors, called ionotropic receptors (Irs), is also involved in olfaction in D. melanogaster. Structural analyses have revealed their similarities to the ionotropic glutamate receptors (iGluRs) with a homology of less than 34% [84,85]. They also share the ion-channel-like characteristic of iGluRs, though the binding site for glutamate is not present [86,87]. The chemosensory apparatus of the larval stage is mainly distributed to the head region, encompassing the dorsal organ (DO), terminal organ (TO), ventral organ (VO), dorsal pharyngeal organ (DPO), dorsal pharyngeal sensilla (DPS), ventral pharyngeal sensilla (VPS), and posterior pharyngeal sensilla (PPS), all existing in pairs [45,88,89,90]. All of the above organs express Irs. A few Irs are also present in the body area that forms the complete larval repertoire together with the receptors of the head region (Table 2) [91]. Contrarily, the distribution of Irs in adult flies is quite diverse (Table 3). In the head region, they are dispersed in the antenna, labral sense organ (LSO), dorsal cibarial sense organ (DCSO), ventral cibarial sense organ (VCSO), and the labellum. The Irs are also expressed in the wings and legs of the fruit fly [91].
Table 2. Ionotropic receptors (Irs) in Drosophila melanogaster (larva).
| DO | TO | VO | DPO/DPS | VPS | PPS | Abdomen |
|---|---|---|---|---|---|---|
| Ir21a | Ir7a | Ir7g | Ir7a | Ir7b | Ir25a | Ir7d |
| Ir25a | Ir7b | Ir25a | Ir7f | Ir7g | Ir76b | Ir7g |
| Ir68a | Ir7d | Ir67a | Ir7g | Ir25a | Ir92a | Ir10a |
| Ir92a | Ir7e | Ir76b | Ir11a | Ir76b | Ir94g | Ir25a |
| Ir93a | Ir7g | Ir25a | Ir100a | Ir68b | ||
| Ir25a | Ir48b | Ir76b | ||||
| Ir56c | Ir48c | Ir85a | ||||
| Ir60c | Ir51b | |||||
| Ir76b | Ir60b | |||||
| Ir94e | Ir60d | |||||
| Ir94h | Ir60e | |||||
| Ir67b | ||||||
| Ir67c | ||||||
| Ir76b | ||||||
| Ir92a | ||||||
| Ir94a | ||||||
| Ir94b | ||||||
| Ir94g | ||||||
| Ir100a |
Outline of larval repertoire of ionotropic receptors (Irs). (Adapted from [91]). DO—Dorsal organ, TO—Terminal organ, VO—Ventral organ, DPO—Dorsal pharyngeal organ, DPS—Dorsal pharyngeal sensilla, VPS—Ventral pharyngeal sensilla, PPS—Posterior pharyngeal sensilla.
The Irs confer odorant responses much like the Ors, but more in-depth studies need to be conducted in this direction. The number of Ir genes in the D. melanogaster genome is 61, in addition to 2 putative pseudogenes. Among these, 17 genes encode the antennal Irs, while the rest of them are translated and scattered all over the fly’s body (both in the larval and adult stages); the latter are called divergent Irs. Amid the antenna, out of 13 Irs, the majority of them sense olfactory cues from amines, aldehydes and evaporative acids, and few Irs are hygrosensory or thermosensory in nature [60,85,91,92]. The remaining four Irs, i.e., Ir8a, Ir25a, Ir76b, and Ir93a are present and function as co-receptors in different amalgamations [93,94]. This paired functioning of Irs with co-receptors suggests that they work in tandem, resulting in ion channels responding to volatile odorant molecules [94,95]. Additionally, the identification of the co-receptor extra loop (CREL), a conserved sequence of the Ir co-receptor ligand-binding domain (LBD) has brought an array of new insights. It has helped in understanding the assembly and stoichiometry of Ir complexes and their intracellular transport in a much-improved way [96]. Besides olfaction, the Irs are also involved in the perception of gustatory stimuli. A group of approximately 35 Irs, the Ir20a clade is translated in all of the taste organs of the fly co-expressed with bitter or sweet Grs [97]. This is explained in detail later, in the gustatory section of the review.
Table 3. Ionotropic receptors (Irs) in Drosophila melanogaster (adult).
| Antenna | LTB | LTP | LSO | VCSO | DCSO | Legs | Wings | Abdomen |
|---|---|---|---|---|---|---|---|---|
| Ir8a | Ir7a | Ir25a | Ir10a | Ir7a | Ir10a | Ir7a | Ir7a | Ir7a |
| Ir21a | Ir7c | Ir56d | Ir20a | Ir11a | Ir20a | Ir7c | Ir7b | Ir7c |
| Ir25a | Ir11a | Ir76b | Ir25a | Ir20a | Ir25a | Ir11a | Ir7c | Ir10a |
| Ir31a | Ir25a | Ir56a | Ir25a | Ir76b | Ir20a | Ir10a | Ir11a | |
| Ir40a | Ir47a | Ir60b | Ir56a | Ir100a | Ir25a | Ir25a | Ir20a | |
| Ir41a | Ir56a | Ir60c | Ir76b | Ir47a | Ir52a | Ir25a | ||
| Ir64a | Ir56b | Ir60d | Ir94a | Ir52a | Ir52b | Ir56a | ||
| Ir68a | Ir56d | Ir67c | Ir94b | Ir52b | Ir52c | Ir76b | ||
| Ir75a | Ir60c | Ir76b | Ir94c | Ir52c | Ir56a | |||
| Ir75b | Ir60d | Ir94a | Ir94h | Ir52d | Ir56d | |||
| Ir75c | Ir76b | Ir94e | Ir56a | Ir60c | ||||
| Ir75d | Ir94b | Ir94f | Ir56d | Ir76b | ||||
| Ir76a | Ir94e | Ir94h | Ir60c | Ir94e | ||||
| Ir76b | Ir100a | Ir67a | ||||||
| Ir84a | Ir76b | |||||||
| Ir92a | Ir94e | |||||||
| Ir93a | Ir94h |
Outline of distribution of ionotropic receptors (Irs) in adult flies. (Adapted from [91,97]). LTB—Labellar taste bristles, LTP—Labellar taste pegs, LSO—Labral sense organ, VCSO—Ventral cibarial sense organ, DCSO—Dorsal cibarial sense organ.
2.4. Odorant Binding Proteins (Obps)
Encoded by a clan of 52 genes, the Obps are found in abundance in D. melanogaster [92]. They are a group of highly divergent pint-sized proteins of 13–28 kDa secreted in the olfactory sensillar lymph [98,99]. The Obps ease the transportation of hydrophobic odorants such as a few food odorants, pheromones, etc. to the Ors [98,100,101]. The Obp76a, also known as LUSH, is essential for the detection of the pheromone cis-vaccenyl acetate (cVA) by Or67d in trichoid sensilla [102]. However, various studies have shown that even in the absence of Obp76a, D. melanogaster can detect cVA, refuting its essentiality [103,104]. Another study involving Obp28a, one of the most abundant Obps in fruit flies, showed that it was not involved in the transportation of odorants [105]. Contrary to these findings, recent work has established that Obp28a is essential for the detection of the floral odorant β-ionone by fruit flies [106]. Thus, these findings of the functioning of different Obps suggest that a much larger picture of the exact role and mechanism of their operation remains to be deciphered.
This entry is adapted from the peer-reviewed paper 10.3390/insects13020142
This entry is offline, you can click here to edit this entry!
