Inflammation constitutes the common pathogenetic substrate for many acute and chronic diseases. It represents the human’s immune system response to various stimuli, such as pathogens, toxic compounds and damaged cells, regulated by a variety of endogenous factors, including cytokines, growth factors and activated cells [
6]. There are two types of inflammation, acute and chronic. While acute inflammation usually refers to a short-term activation of the immune system as a response to an external trigger, chronic inflammation may occur in the absence of any specific stimuli, resulting in the development of many chronic diseases. It is now well established that several chronic disorders, such as connective tissue and inflammatory bowel diseases, diabetes mellitus, cancer and cardiovascular disorders share, to a lesser or greater degree, common pathogenetic mechanisms involving inflammation [
7].
There is now an emerging body of evidence to support the anti-inflammatory activity of Chios mastiha. This anti-inflammatory action seems to be performed via the inhibition of the production of pro-inflammatory substances. In particular, administration of both solid and liquid types of mastiha seems to inhibit prostaglandin secretion along with inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 expression by macrophages at both protein and mRNA level in animal experimental models [
8]. In vitro, mastiha blocks the expression of the adhesion molecules VCAM-1 and ICAM-1 by TNF-alpha-stimulated human aortic endothelial cells, thereby interfering in endothelial activation that is recognized as the primary event of the atherosclerotic process [
9].
Data derived by human studies are also in the same direction (
Table 2). In a small clinical study including 10 patients with mild or moderately active Crohn’s disease recruited to treatment with mastic caps for 4 weeks (2.2 g/day), a significant decrease in the activity index of the disease and the plasma levels of interleukin-6 and CRP compared to baseline was observed, while no significant side effects were reported [
10]. In the same patient cohort, a remarkable reduction in TNF-a secretion following treatment with mastic caps was later reported, suggesting an additional inhibitory mechanism of monocyte chemotaxis, thus providing more support to the role of mastiha as immune system regulator [
11].
Table 2. Human studies assessing mastic’s effects.
Based on the findings of this pilot study, in 2019, Papada et al. designed and performed a randomized controlled trial to further investigate the impact of mastiha on patients with inflammatory bowel disease. A total of 60 patients were randomly assigned to receive either mastiha (2.8 g/day) or placebo for 3 months adjunct to standard medication. Patients treated with mastiha had a significant decrease in faecal lysozyme compared to patients on placebo, indicative of lower disease activity. In addition to this, a significant improvement in Inflammatory Bowel Disease Questionnaire scores reflecting a beneficial effect on patients’ quality of life was observed in the mastiha arm compared to the baseline [
12]. When the same protocol was applied to 68 patients with inactive inflammatory bowel disease for 6 months, in contrast to controls, patients allocated to mastiha as add-on treatment to standard medication presented no increase in interleukin-6 or in faecal biomarkers calprotectin and lactoferrin, which are neutrophil-derived proteins whose concentrations typically rise in patients with gastrointestinal mucosal inflammation [
14]. Recent data support that mastiha treatment interferes in the regulation of Th17 cell function and differentiation, resulting in increased serum levels of interleukin-17A that is considered to play a protective role in the development and relapse of inflammatory bowel disease [
15].
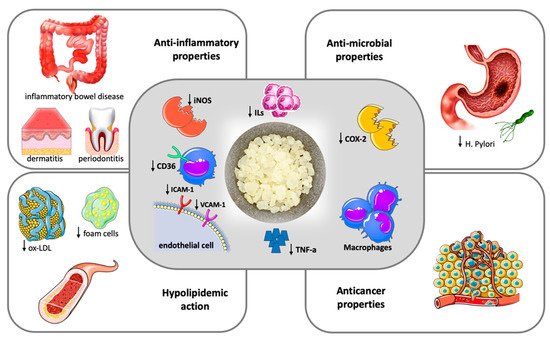
Figure 1 summarizes the most important pathogenetic and clinical effects of mastic.
Figure 1. Therapeutic potentials of Chios Mastic.
Chios mastic exerts anti-inflammatory and antioxidative properties (central frame). Anti-inflammatory action is attributed to the inhibition of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 expression by macrophages and the blockage of the expression of the adhesion molecules VCAM-1 and ICAM-1 by TNF-alpha stimulated endothelial cells, ultimately resulting in reduced Tumor Necrosis-alpha (TNA-a) and inflammatory interleukins (ILs) production. The antioxidative properties are mainly driven by a downregulation of CD36 expression in macrophages along with an increase in the intracellular antioxidant glutathione levels. The clinical effects of mastic (outer frames) represent the result of anti-inflammatory and anti-oxidant action and include a hypolipidemic action with a decrease in oxidized-LDL (ox-LDL) particles and foam cell formation, beneficial effects in inflammatory bowel disease, dermatitis and periodontal inflammation, antimicrobial and anticancer properties.
Mastic demonstrates a protective effect on intestinal epithelial cells, largely determined by its anti-inflammatory and antioxidant properties [
25]. This action has been more thoroughly investigated in inflammatory bowel diseases, where mastic has been found to decrease the cytokines tumor necrosis factor-a, malonaldehyde, intercellular adhesion molecule-1 (ICAM-1) and interleukin -6, -8 and -10, both in preclinical and clinical studies; thus, efficiently inhibiting intestinal damage [
26,
27]. In addition, mastiha supplementation promotes a partial but respectable recovery of microbial diversity, acting as a natural probiotic factor [
28]. A randomized controlled trial including 148 subjects showed that mastic, at a dose of 350 mg three times daily, significantly improved symptoms related with functional dyspepsia after 3 weeks of treatment [
16].
It has also been found that mastic reduces liver enzymes and improves hepatic steatosis and collagen content in experimental models with non-alcoholic fatty liver disease (NAFLD) [
28]. Considering that oxidation and inflammation dominate the pathogenetic substrate of NAFLD, the MAST4HEALTH randomized clinical trial reported a significant improvement in total antioxidant status of obese patients with NAFLD treated with mastiha for a 6 month period [
17]. Several mastic compounds, including oleanonic acid, oleanolic acid and gallic acid act as modulators of peroxisome proliferator-activated receptors (PPARs), which are recognized as regulators of glucose and lipid metabolism, inflammation and fibrosis progression in the liver, playing a crucial role in the development of NAFLD [
29].
There is an accumulating body of evidence suggesting that the topical application of mastic ointment attenuates inflammatory and/or pruritic responses in animal experimental models of allergic dermatitis [
30]. This action is once again attributed to the anti-inflammatory properties of mastic and, particularly, to the drastic reduction of cytokine production. Clinical data derived from randomized controlled trials also suggest that mastic exhibits a beneficial effect on wound healing. Higher healing rates of episiotomy wound healing were observed in 73 women who were treated for three days postpartum with mastic oleoresin, which was administered through smoking of the wound [
18]. These data are consistent with findings provided by animal studies that demonstrate a favorable action of mastic oil on the healing of wounds caused by laser burns [
31].
The aforementioned data undoubtedly support that mastic possesses anti-inflammatory properties. On the other hand, with the exception of inflammatory bowel disorders, data regarding the potential anti-inflammatory effect of mastiha on other systemic inflammatory disorders are scarce. In these terms, large-scale, well-designed clinical trials involving patients with common inflammatory disorders are yet to be performed in order to establish the anti-inflammatory function of mastiha.
2. Anti-Oxidant Properties
Oxidative stress denotes an imbalance between the production of reactive oxygen species (ROS) and the antioxidant defense of a biological system [
32]. Free radicals of oxygen produced by the cellular metabolism play an integral role in the modulation of cell signaling, differentiation and proliferation.
Excessive production of ROS may, however, cause oxidative stress, which is responsible for harmful changes on DNA, RNA protein and lipids, and associated with an increased risk for several chronic and systemic disorders—namely cardiovascular disease, systemic inflammatory disorders and cancer [
33]. To that end, natural antioxidants have gained substantial scientific interest, driven by their numerous health benefits.
The oxidization of LDL is representative of changes induced by oxidative stress. After multiple modifications taking place on LDL particles, the oxidized LDL acquire atherogenic and pro-inflammatory properties, drastically contributing to the development of atherosclerosis [
34]. Investigating the antioxidant potential of several gums and resins in vitro, Andrikopoulos et al. demonstrated that mastiha was the most effective one in preventing human LDL oxidation [
35]. This action was mainly attributed to the hydromethanolic component of mastiha, with triterpenes and hydroxynaphthoquinones demonstrating LDL protective activity as well.
Mastiha attenuates cellular superoxide production by downregulating NADPH oxidase through the inhibition of protein kinase C pathways, a process that is possibly triggered by TNF-a, again underlining the close interaction between inflammation and oxidative stress [
19]. Protein kinase C is known to hold a key position in a variety of cellular signaling pathways, being reversibly modulated by ROS owing to its unique structural feature that is susceptible to oxidative modification [
36]. Inhibition of such pathways by mastiha, particularly by its triterpenes, leads to an increase in the intracellular antioxidant glutathione levels along in macrophages along with a downregulation of CD36 expression [
37]. The latter are recognized as the oxLDL receptors in macrophages and keep a central role in the foam cell formation atherogenesis, upregulated in the presence of oxLDL and interleukin-4 [
38].
3. Anti-Atherogenic Properties
The anti-atherogenic effects of Chios mastic are associated with its anti-inflammatory and antioxidant properties. As aforementioned, beyond intracellular antioxidant glutathione enhancement, it is suggested that mastic triterpenes exhibit their antioxidant effect by downregulating CD36 expression in macrophages, thus preventing oxLDL uptake that promotes the formation and accumulation of foam cells at sites of vascular endothelial dysfunction in both early and late steps of atherosclerosis. In animal experimental models, mastic treatment has been found to possess anti-ischemic properties, leading to a reduction in infarct size [
39]. However, clinical evidence on the anti-atherogenic or cardioprotective effects of mastic is limited, and mainly derived from studies assessing surrogate markers of atherosclerosis.
A remarkable reduction in lipid levels was demonstrated in a clinical trial including 133 patients, who were randomly assigned to receive either high-dose (5 g/daily) or low-dose (mastic solution) mastiha treatment. More specifically, patients on high-dose mastic presented a significant decrease in serum total cholesterol, LDL, total cholesterol/HDL ratio, lipoprotein a, apolipoprotein A-1 and apolipoprotein B levels after an 18-month treatment period. Moreover, a decrease in liver enzyme values in the same group was observed [
40]. Similar findings were reported in 60 patients with inflammatory bowel disease, randomized to either mastic treatment (2.8 g/day) or placebo for 3 months while receiving standard treatment for their disease. Treatment with mastic was associated to a significant reduction in oxLDL levels along with oxLDL/LDL and oxLDL/HDL ratios [
13].
The ‘Chios-Mastiha’ trial included 156 patients with total cholesterol values > 200 mg/dl who were randomly allocated to placebo, 1 g/day of crude mastic (total mastic group), 1 g/day of polymer free mastic or 2 g/day of powder mastic. Of note, after 8 weeks of treatment, only patients treated with 1 g of crude mastic per day exhibited a significant reduction in total cholesterol values of about 11.5 mg/dl compared to baseline, with this effect being more pronounced in overweight and obese patients. This was accompanied by a decrease in free plasma glucose levels of about 4.5 mg/dl. Nonetheless, these findings should be interpreted with caution, given that no effects on LDL, HDL, triglycerides or CRP were observed [
20]. In a different concept, compared to placebo, mastiha treatment exerted favorable effects on peripheral and aortic hemodynamics, as assessed by non-invasive aortic blood pressure measurements and aortic augmentation index among 13 hypertensive patients [
21].
These data imply that mastiha may display cardioprotective effects driven by its lipid-lowering action. For the moment, there are a lack of studies with hard cardiovascular end-points, which are expected to shed more light on the cardioprotective potential of mastiha in the future.
4. Conclusions
Despite the great progress that has been made on human health and the remarkable ongoing development in medical products, there is an increasing interest, nowadays, for natural supplements that may exert beneficial health effects. In this context, the existing data suggest that Chios mastic possesses anti-inflammatory and anti-oxidative properties which could be utilized in the treatment of multiple disorders. Given the emerging antimicrobial resistance trends, the establishment of mastic’s antibacterial efficacy could support its introduction as adjunct therapy in the management of various infectious diseases. Moreover, the antilipidemic activity of mastic that has been observed prompts the conduction of clinical trials with cardiovascular endpoints to assess its possible value in the management of cardiovascular disorders. Another point that should be underlined is that, to date, no significant adverse effects associated with human consumption of mastic have been reported.
Overall, Chios mastic gathers many favorable properties that could justify its therapeutic use for a variety of human diseases. Most of the research data, however, derive from studies on animal experimental models or studies performed in vitro, while the number human studies in this direction are, for the moment, limited. There is, therefore, need for further clinical research in order to assess the therapeutic potential of mastic in different disorders and to unravel its complex mechanism of action.