Human and animal health are intimately connected. This idea has been known for more than a century but now it has gained special importance because of the increasing threat from zoonoses. Zoonosis is defined as any infection naturally transmissible from vertebrate animals to humans. As the frequency and prevalence of zoonotic diseases increase worldwide, they become a real threat to public health. In addition, many of the newly discovered diseases have a zoonotic origin. Due to globalization and urbanization, some of these diseases have already spread all over the world, caused by the international flow of goods, people, and animals. However, special attention should be paid to farm animals since, apart from the direct contact, humans consume their products, such as meat, eggs, and milk. Therefore, zoonoses such as salmonellosis, campylobacteriosis, tuberculosis, swine and avian influenza, Q fever, brucellosis, Shiga-toxic Escherichia coli (STEC) infections, and listeriosis are crucial for both veterinary and human medicine. Consequently, in the suspicion of any zoonoses outbreak, the medical and veterinary services should closely cooperate to protect the public health.
- One Health
- zoonotic pathogens
- foodborne diseases
1. Background
| Disease | Aetiological Agent | Human Symptoms | Transmission Route | Epidemiology | References |
|---|---|---|---|---|---|
| Q fever | Coxiellaburnetti | Self-limited febrile illness, pneumonia, hepatitis, and endocarditis | Inhalation of aerosolized bacteria, ingestion, transfusion of blood, and sexual transmission | EU—950 human cases in 2019 USA—178 human cases in 2019 |
[5] [6] [15] |
| Brucellosis | Brucellaabortus, B. melitensis, B. canis, B. suis |
Systematic syndrome (fever, sweat, chills, and fatigue), located presentations (epididymoorchitis and spondylodiscitis), neurobrucellosis, and endocarditis | Contaminated food and dairy products, occupational contact, and inhalation | World—around 500,000 human cases per year EU—310 human cases in 2019 USA—80–120 cases annually |
[5] [6] [16] |
| Tuberculosis | Mycobacterium bovis M. caprae |
Generalized symptoms (fever, fatigue, arthralgia, and muscle pain), respiratory and cardiac complications, hepatitis, osteoarthritis, central nervous system dysfunction, and orchitis/epididymitis | Inhalation of aerosol, infected milk, dairy products, and meat | EU—147 human cases in 2019 USA—7174 human cases in 2020 |
[5] [17] [18] |
| Trichinellosis | Trichinella sp. | Diarrhea, abdominal pain at first, fever, myalgia, myocarditis, facial oedemas, and encephalitis | Ingestion of raw or undercooked muscle tissue containing encysted larvae | EU—96 human cases in 2019 USA—90 human cases during 2008–2012 |
[5] [6] [19] |
| Yersiniosis | Yersinia enterocolitica, Y. pseudotuberculosis |
Fever, vomiting, abdominal pain, and bloody diarrhea | Eating raw or undercooked pork; ingestion of dairy products, seafood, and vegetables; or drinking contaminated water | EU—6961 human cases USA—nearly 117,000 illnesses per year |
[5] [6] [20] |
| Swine influenza |
Swine influenza virus (SIV) |
Sneezing, coughing, difficult breathing, fever, lethargy, and decreased appetite | Contact with respiratory discharges or inhalation of exhalated aerosol by sick pig | No specific epidemiological data available, spread worldwide |
[21] [22] [23] |
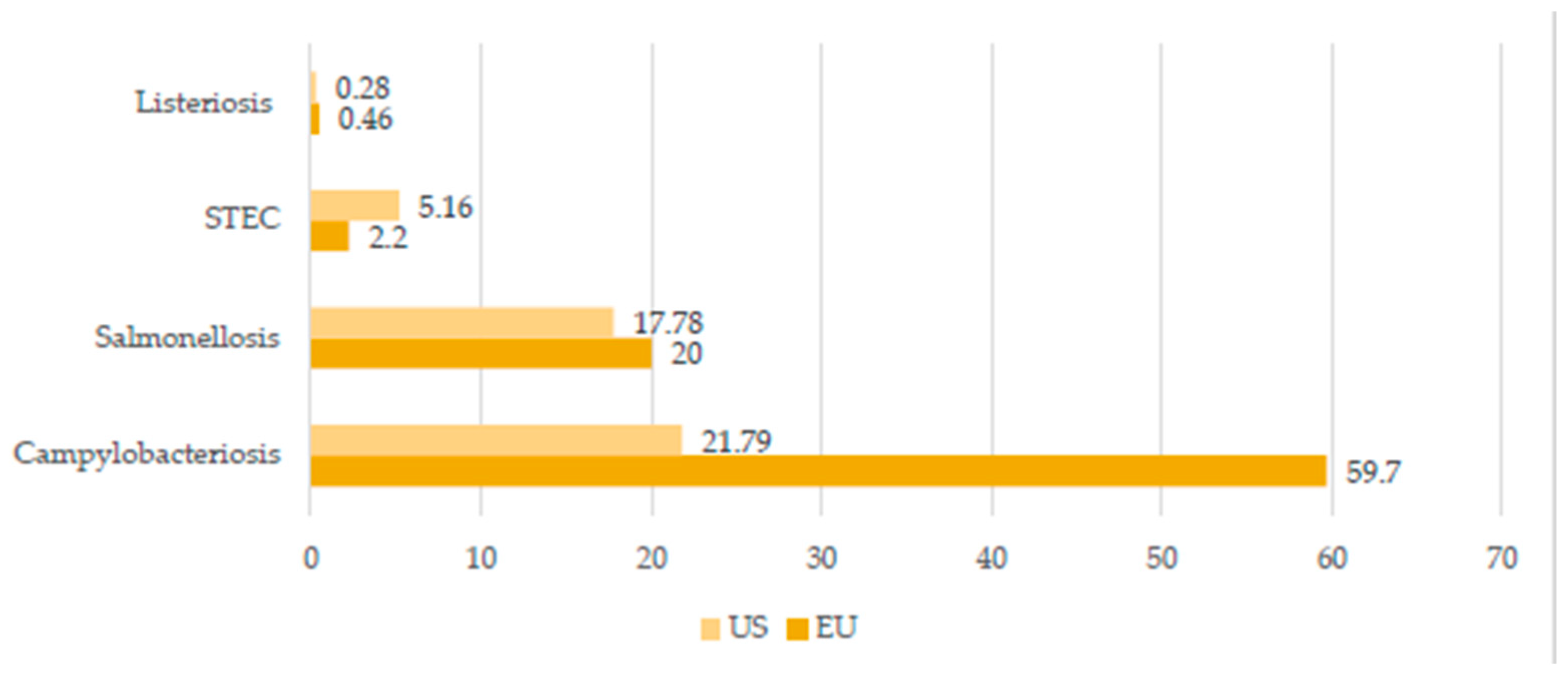
| Salmonellosis | Salmonella sp. | Acute enterocolitis accompanied by inflammatory diarrhea, abdominal pain, fever, nausea, and vomiting | Ingestion of uncooked contaminated foods (eggs, milk, and meat), drinking contaminated water, direct contact with infected animals, their feces and environment, and human-to-human transmission through fecal–oral route | EU—87,923 human cases in 2019 USA—about 1.35 million human illnesses per year Sub-Saharan Africa—535,500 cases of non-typhoidal salmonellosis in 2019 |
[5] [6] [24] [25] |
2. Q Fever
3. Brucellosis
4. Tuberculosis Caused by Mycobacterium bovis and Mycobacterium caprae
5. Salmonellosis
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/idr14010008
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423); United Nations: New York, NY, USA, 2019.
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021.
- World Organization for Animal Health Official Website. Available online: https://www.oie.int/en/what-we-do/global-initiatives/one-health/ (accessed on 25 November 2021).
- Pomorska-Mól, M.; Włodarek, J.; Gogulski, M.; Rybska, M. Review: SARS-CoV-2 Infection in farmed minks—An overview of current knowledge on occurrence, disease and epidemiology. Animal 2021, 15, 100272.
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report; EFS2; 2021; Volume 19, pp. 1–286. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2021.6406 (accessed on 5 December 2021).
- Centers for Disease Control and Prevention Official Website. Available online: https://www.cdc.gov/onehealth/pdfs/us-ohzdp-report-508.pdf (accessed on 25 November 2021).
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System, 2019 Annual Tables of Infectious Disease Data; CDC Division of Health Informatics and Surveillance: Atlanta, GA, USA, 2021.
- Kemunto, N.; Mogoa, E.; Osoro, E.; Bitek, A.; Kariuki Njenga, M.; Thumbi, S.M. Zoonotic disease research in East Africa. BMC Infect. Dis. 2018, 18, 545.
- Liu, Q.; Cao, L.; Zhu, X.-Q. Major emerging and re-emerging zoonoses in China: A matter of global health and socioeconomic development for 1.3 billion. Int. J. Infect. Dis. 2014, 25, 65–72.
- Wille, M.; Geoghegan, J.L.; Holmes, E.C. How accurately can we assess zoonotic risk? PLoS Biol. 2021, 19, e3001135.
- Belay, E.D.; Kile, J.C.; Hall, A.J.; Barton-Behravesh, C.; Parsons, M.B.; Salyer, S.; Walke, H. Zoonotic disease programs for enhancing global health security. Emerg. Infect. Dis. 2017, 23, S65.
- Acharya, K.P.; Acharya, N.; Phuyal, S.; Upadhyaya, M.; Lasee, S. One-Health approach: A best possible way to control rabies. One Health 2020, 10, 100161.
- Kruse, H.; Kirkemo, A.-M.; Handeland, K. Wildlife as source of zoonotic infections. Emerg. Infect. Dis. 2004, 10, 2067–2072.
- Rocklöv, J.; Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020, 21, 479–483.
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190.
- Amjadi, O.; Rafiei, A.; Mardani, M.; Zafari, P.; Zarifian, A. A review of the immunopathogenesis of brucellosis. Infect. Dis. 2019, 51, 321–333.
- Kozińska, M.; Krajewska-Wędzina, M.; Augustynowicz-Kopeć, E. Mycobacterium caprae—the first case of the human infection in Poland. Ann. Agric. Environ. Med. 2020, 27, 151–153.
- World Health Organization; Food and Agriculture Organization of the United Nations. World Organisation for Animal Health Roadmap for Zoonotic Tuberculosis; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151304-3.
- Bilska-Zając, E.; Różycki, M.; Korpysa-Dzirba, W.; Bełcik, A.; Ziętek-Barszcz, A.; Włodarczyk-Ramus, M.; Gontarczyk, A.; Cencek, T. Trichinella outbreaks on pig farms in Poland in 2012–2020. Pathogens 2021, 10, 1504.
- Laukkanen-Ninios, R.; Fredriksson-Ahomaa, M.; Maijala, R.; Korkeala, H. High prevalence of pathogenic Yersinia enterocolitica in pig cheeks. Food Microbiol. 2014, 43, 50–52.
- Smith, G.J.D.; Vijaykrishna, D.; Bahl, J.; Lycett, S.J.; Worobey, M.; Pybus, O.G.; Ma, S.K.; Cheung, C.L.; Raghwani, J.; Bhatt, S.; et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 2009, 459, 1122–1125.
- Ito, T.; Couceiro, J.N.S.S.; Kelm, S.; Baum, L.G.; Krauss, S.; Castrucci, M.R.; Donatelli, I.; Kida, H.; Paulson, J.C.; Webster, R.G.; et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 1998, 72, 7367–7373.
- Myers, K.P.; Olsen, C.W.; Setterquist, S.F.; Capuano, A.W.; Donham, K.J.; Thacker, E.L.; Merchant, J.A.; Gray, G.C. Are swine workers in the United States at increased risk of infection with zoonotic influenza virus? Clin. Infect. Dis. 2006, 42, 14–20.
- Popa, G.L.; Popa, M.I. Salmonella spp. infectio—A continuous threat worldwide. Germs 2021, 11, 88–96.
- Roadmap to Reducing Salmonella Driving Change through Science-Based Policy; Food Safety the U.S. Department of Agriculture: Washington, DC, USA, 2020.
- Derrick, E.H. “Q” fever, a new fever entity: Clinical features, diagnosis and laboratory investigation. Med. J. Aust. 1937, 2, 281–299.
- Miller, H.K.; Priestley, R.A.; Kersh, G.J. Transmission of Coxiella burnetii by ingestion in mice. Epidemiol. Infect. 2020, 148, 1–8.
- Pechstein, J.; Schulze-Luehrmann, J.; Lührmann, A. Coxiella burnetii as a useful tool to investigate bacteria-friendly host cell compartments. Int. J. Med. Microbiol. Suppl. 2018, 308, 77–83.
- España, P.P.; Uranga, A.; Cillóniz, C.; Torres, A. Q fever (Coxiella burnetii). Semin. Respir. Crit. Care. Med. 2020, 41, 509–521.
- Kersh, G.J.; Priestley, R.; Massung, R.F. Stability of Coxiella burnetii in stored human blood: C. burnetii stability in blood. Transfusion 2013, 53, 1493–1496.
- Signs, K.A.; Stobierski, M.G.; Gandhi, T.N. Q fever cluster among raw milk drinkers in Michigan, 2011. Clin. Infect. Dis. 2012, 55, 1387–1389.
- Angelakis, E.; Raoult, D. Q fever. Vet. Microbiol. 2010, 140, 297–309.
- Epelboin, L.; Chesnais, C.; Boulle, C.; Drogoul, A.-S.; Raoult, D.; Djossou, F.; Mahamat, A. Q fever pneumonia in French Guiana: Prevalence, Risk Factors, and Prognostic Score. Clin. Infect. Dis. 2012, 55, 67–74.
- Galińska, E.M.; Zagórski, J. Brucellosis in humans—Etiology, diagnostics, clinical forms. Ann. Agric. Environ. Med. 2013, 20, 6.
- Franco, M.P.; Mulder, M.; Gilman, R.H.; Smits, H.L. Human brucellosis. Lancet Infect. Dis. 2007, 7, 775–786.
- Głowacka, P.; Żakowska, D.; Naylor, K.; Niemcewicz, M.; Bielawska-Drózd, A. Brucella—virulence factors, pathogenesis and treatment. Pol. J. Microbiol. 2018, 67, 151–161.
- Rubach, M.P.; Halliday, J.E.B.; Cleaveland, S.; Crump, J.A. Brucellosis in low-income and middle-income countries. Curr. Opin. Infect. Dis. 2013, 26, 404–412.
- Tuon, F.F.; Gondolfo, R.B.; Cerchiari, N. Human-to-human transmission of Brucella—A systematic review. Trop. Med. Int. Health 2017, 22, 539–546.
- Hull, N.C.; Schumaker, B.A. Comparisons of Brucellosis between human and veterinary medicine. Infect. Ecol. Epidemiol. 2018, 8, 1500846.
- Awah-Ndukum, J.; Mouiche, M.M.M.; Kouonmo-Ngnoyum, L.; Bayang, H.N.; Manchang, T.K.; Poueme, R.S.N.; Kouamo, J.; Ngu-Ngwa, V.; Assana, E.; Feussom, K.J.M.; et al. Seroprevalence and risk factors of brucellosis among slaughtered indigenous cattle, abattoir personnel and pregnant women in Ngaoundéré, Cameroon. BMC Infect. Dis. 2018, 18, 611.
- Mai, H.M.; Irons, P.C.; Kabir, J.; Thompson, P.N. A large seroprevalence survey of brucellosis in cattle herds under diverse production systems in Northern Nigeria. BMC Vet. Res. 2012, 8, 144.
- Zheng, R.; Xie, S.; Lu, X.; Sun, L.; Zhou, Y.; Zhang, Y.; Wang, K. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. BioMed Res. Int. 2018, 2018, 5712920.
- Prodinger, W.M.; Indra, A.; Koksalan, O.K.; Kilicaslan, Z.; Richter, E. Mycobacterium caprae infection in humans. Expert Rev. Anti Infect. Ther. 2014, 12, 1501–1513.
- Kaneene, J.B.; Miller, R.; de Kantor, I.N.; Thoen, C.O. Tuberculosis in wild animals. Int. J. Tuberc. Lung Dis. 2010, 14, 1508–1512.
- Khan, M.; Islam, M.M.; Ferdous, J.; Alam, M. An overview on epidemiology of tuberculosis. Mymensingh Med. J. 2019, 28, 259–266.
- El-Sayed, A.; El-Shannat, S.; Kamel, M.; Castañeda-Vazquez, M.A.; Castañeda-Vazquez, H. Molecular epidemiology of Mycobacterium bovis in humans and cattle. Zoonoses Public Health 2016, 63, 251–264.
- World Health Organization. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019.
- Michel, A.L.; Müller, B.; van Helden, P.D. Mycobacterium bovis at the animal–human interface: A problem, or not? Vet. Microbiol. 2010, 140, 371–381.
- Grange, J.M. Mycobacterium bovis infection in human beings. Tuberculosis 2001, 81, 71–77.
- Jalava, K.; Jones, J.A.; Goodchild, T.; Clifton-Hadley, R.; Mitchell, A.; Story, A.; Watson, J.M. No increase in human cases of Mycobacterium bovis disease despite resurgence of infections in cattle in the United Kingdom. Epidemiol. Infect. 2007, 135, 40–45.
- Palmer, M.V. Mycobacterium bovis: Characteristics of wildlife reservoir hosts. Transbound. Emerg. Dis. 2013, 60, 1–13.
- De Lisle, G.W.; Schlundt, J.; Schmitt, S.M.; O’Brien, D.J. Tuberculosis in free-ranging wildlife: Detection, diagnosis and management. Rev. Sci. Tech. OIE 2002, 21, 317–334.
- O’Reilly, L.M.; Daborn, C.J. The epidemiology of Mycobacterium bovis infections in animals and man: A review. Tuberc. Lung Dis. 1995, 76, 1–46.
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521.
- Li, S.; He, Y.; Mann, D.A.; Deng, X. Global spread of Salmonella enteritidis via centralized sourcing and international trade of poultry breeding stocks. Nat. Commun. 2021, 12, 5109.
- World Health Organization Official Website. Influenza (Avian and Other Zoonotic). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(avian-and-other-zoonotic) (accessed on 25 November 2021).
- Kurtz, J.R.; Goggins, J.A.; McLachlan, J.B. Salmonella infection: Interplay between the bacteria and host immune system. Immunol. Lett. 2017, 190, 42–50.
