SARS-CoV-2, a virus of the family of
Betacoronaviridae, is the etiological agent of COVID-19 disease, a severe illness with the first official case described in December 2019 in Wuhan, China, and currently verified as being responsible for about 4.8 million deaths [
1,
2]. Symptoms and clinical manifestations are common to systemic diseases and include cough, dyspnea, fever, anosmia, mild to severe pneumonitis with diffuse alveolar damage, and acute respiratory distress syndrome, potentially leading to death [
3,
4]. The progressive spread of SARS-CoV-2 all over the world, the related disease severity, and the impact on everyday life and economics have stimulated the development of vaccines with a high safety profile to limit the rising infection rates [
5,
6]. As it stands, different types of vaccines manufactured by pharmaceutical giants are now authorized for administration, including the best known BNT162b2, ChAdOx1, and RNA-1273 vaccines. Despite the rise of new SARS-CoV-2 variants, BNT162b2 seems to induce a powerful immune response comparable to that of the original virus [
7]. The progressive increase in the number of people who have undergone vaccination has revealed common side effects associated with the procedure. Adverse events include fever, myalgias, pain at the injection site, hypercoagulability states, elevated risk of HSV infection, acute myocarditis, appendicitis, and a copious list of unconventional and usual reactions [
5]. Post-COVID-19 vaccine lymphadenopathy is a common adverse event occurring in about 3–16% of patients regardless of the vaccine type administered [
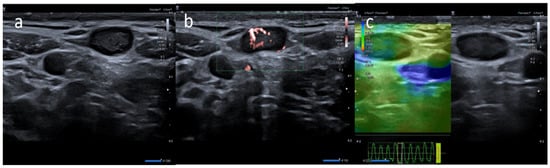
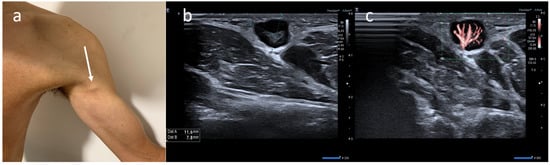
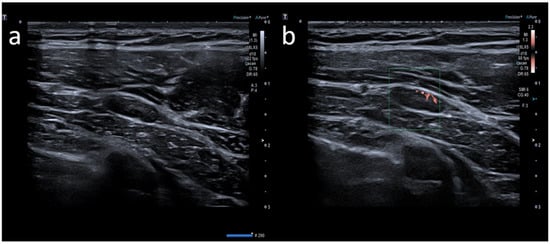
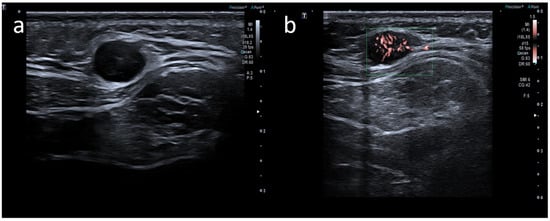
5]. Generally, lymph node enlargement involves axillary and/or supraclavicular nodal stations ipsilateral to the intramuscular injection site [
8,
9,
10,
11,
12,
13,
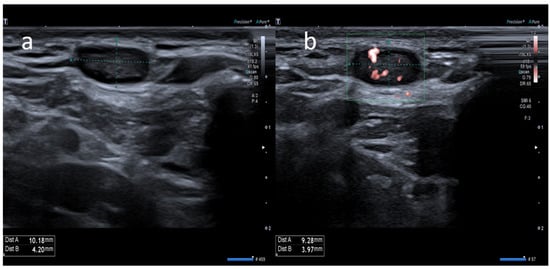
14]. Ultrasound (US) examination is a first-line imaging modality for evaluating morphologic lymph nodes due to its common accessibility and clear depiction of soft tissues and superficial structures provided by high-frequency linear probes [
12,
13]. Ultrasound examination has already played a central role in the pandemic for the management of patients with pulmonary involvement and for the selection of those in need of chest computed tomography (CT) [
14,
15,
16]. Sometimes, benign post-anti-COVID 19 vaccination lymphadenopathies may show “nonreactive” features and may mimic severe pathologies with poorer prognosis [
12,
13]. The diagnostic overlap between the US features of lymph node involvement in benign and malignant disease is extremely relevant in the follow-up of neoplastic patients [
17,
18]. In this setting, research into the peculiar characteristics associated with post-anti-COVID19 vaccine lymphadenopathy may allow the avoidance of worthless follow-up, expansive second-level diagnostic imaging, and invasive diagnostic procedures for selected patients. In this study, we describe the incidence and US features of post-anti-COVID-19 vaccine lymphadenopathies in atypical sites to clarify their potential role in patients’ diagnostic work-up and follow-up strategies.