The high conductivity of graphene material (or its derivatives) and its very large surface area enhance the direct electron transfer, improving non-enzymatic electrochemical sensors sensitivity and its other characteristics. The offered large pores facilitate analyte transport enabling glucose detection even at very low concentration values. Herein classified the enzymeless graphene-based glucose electrocatalysts’ synthesis methods that have been followed into the last few years into four main categories: (i) direct growth of graphene (or oxides) on metallic substrates, (ii) in-situ growth of metallic nanoparticles into graphene (or oxides) matrix, (iii) laser-induced graphene electrodes and (iv) polymer functionalized graphene (or oxides) electrodes. The increment of the specific surface area and the high degree reduction of the electrode internal resistance were recognized as their common targets.
- graphene-based nanomaterials
- synthesis
- reduced graphene oxide
- glucose electrooxidation mechanism
- electrochemical sensor
- cobalt oxide nanomaterial
- copper oxide nanomaterial
- nickel oxide nanomaterial
- direct growth
- in-situ growth
- laser-induced
- polymer functi
1. Direct Growth (or Deposition) of Graphene (or Its Oxides) onto Metallic Substrates
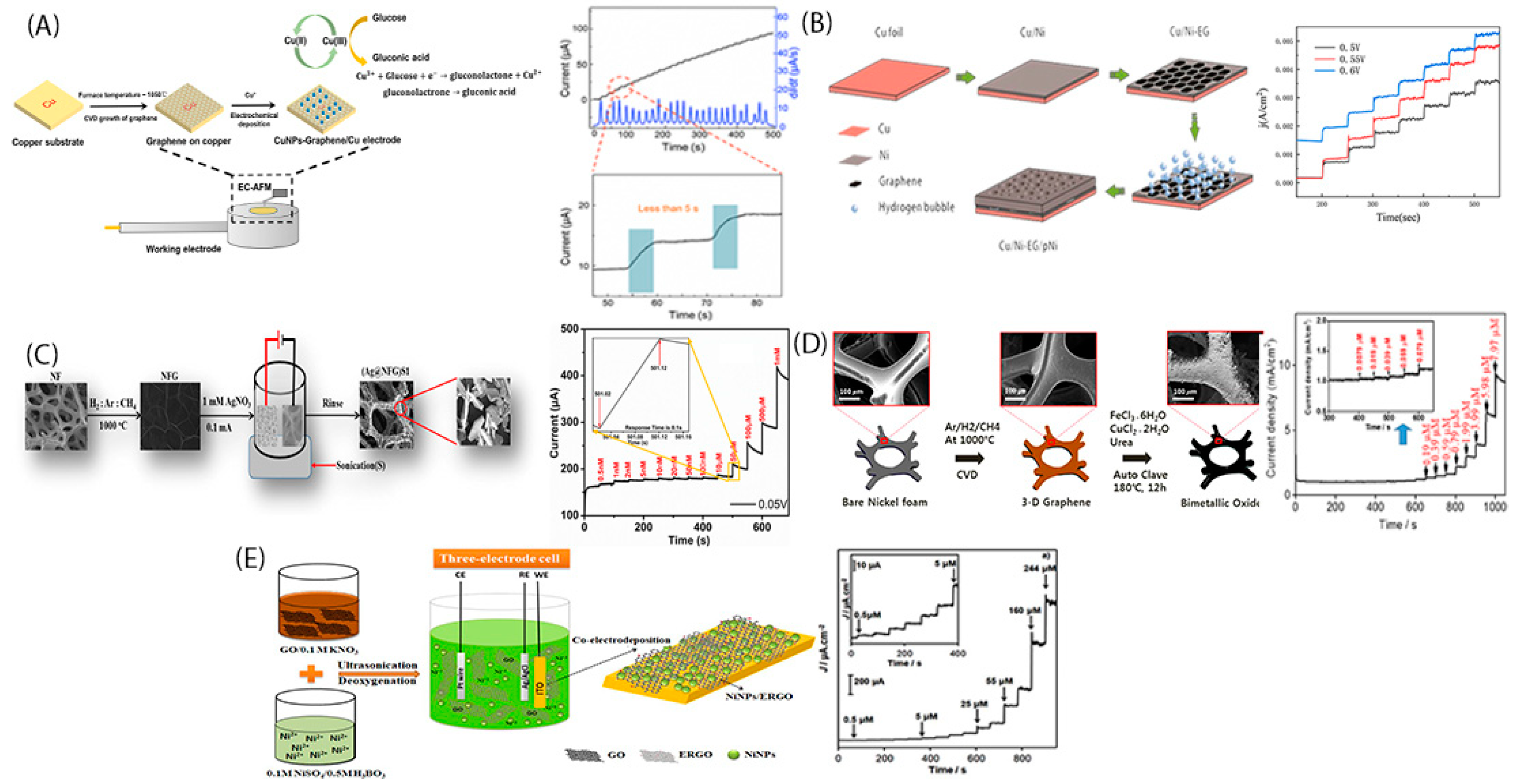
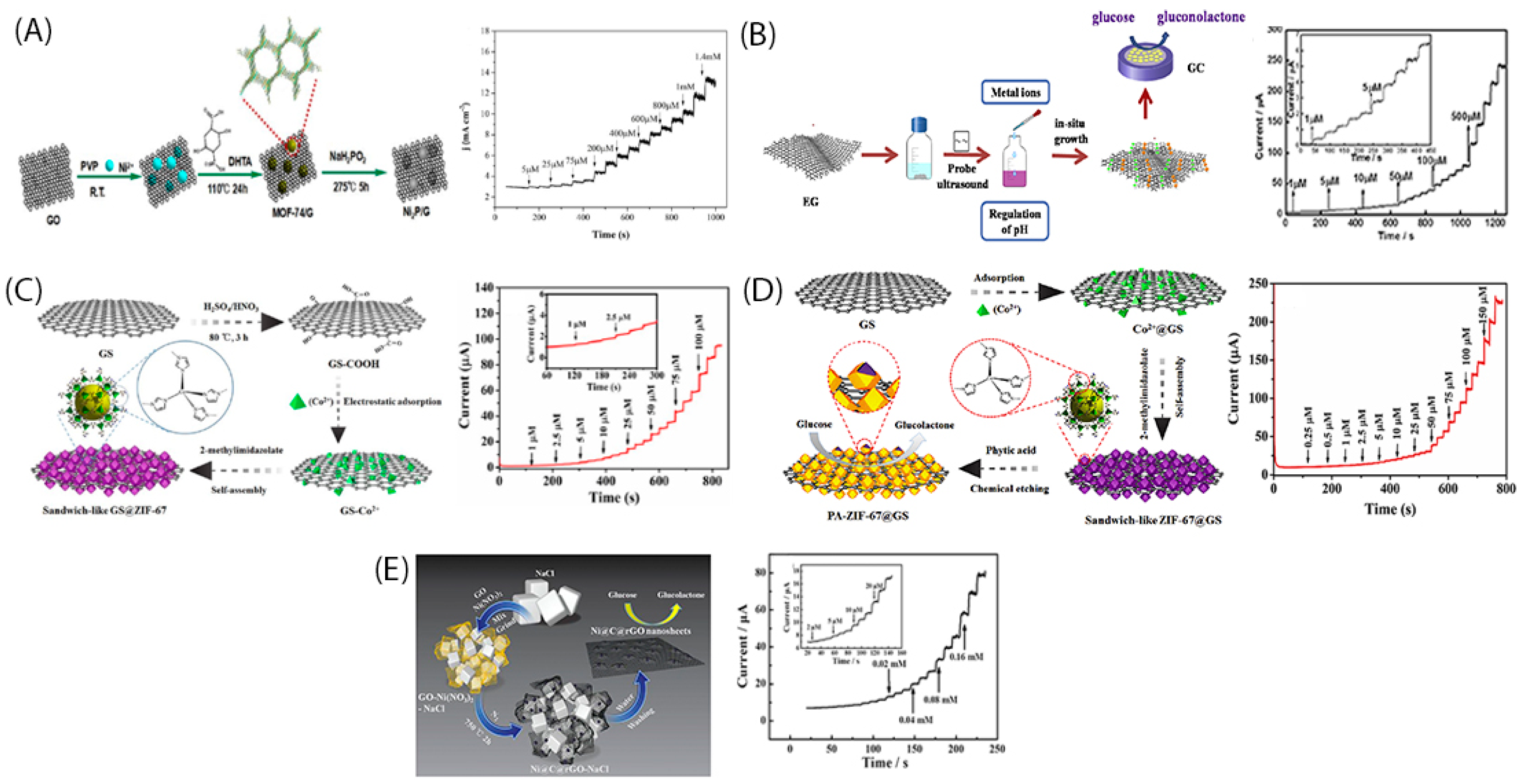
2. In-Situ Growth of Metallic Nanoparticles into Graphene Nanosheets
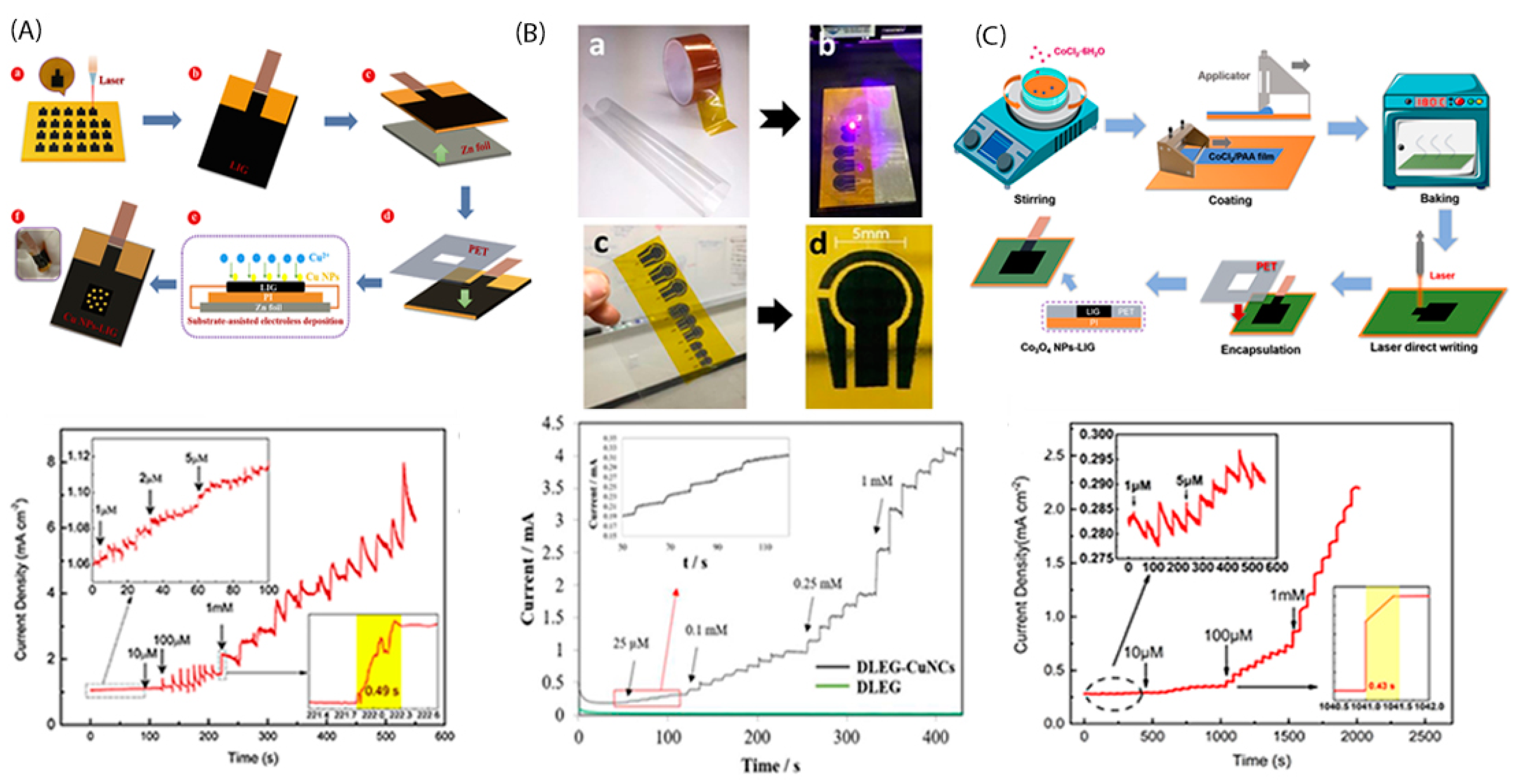
3. Laser-Induced Graphene-Based Electrode Synthesis and Functionalization
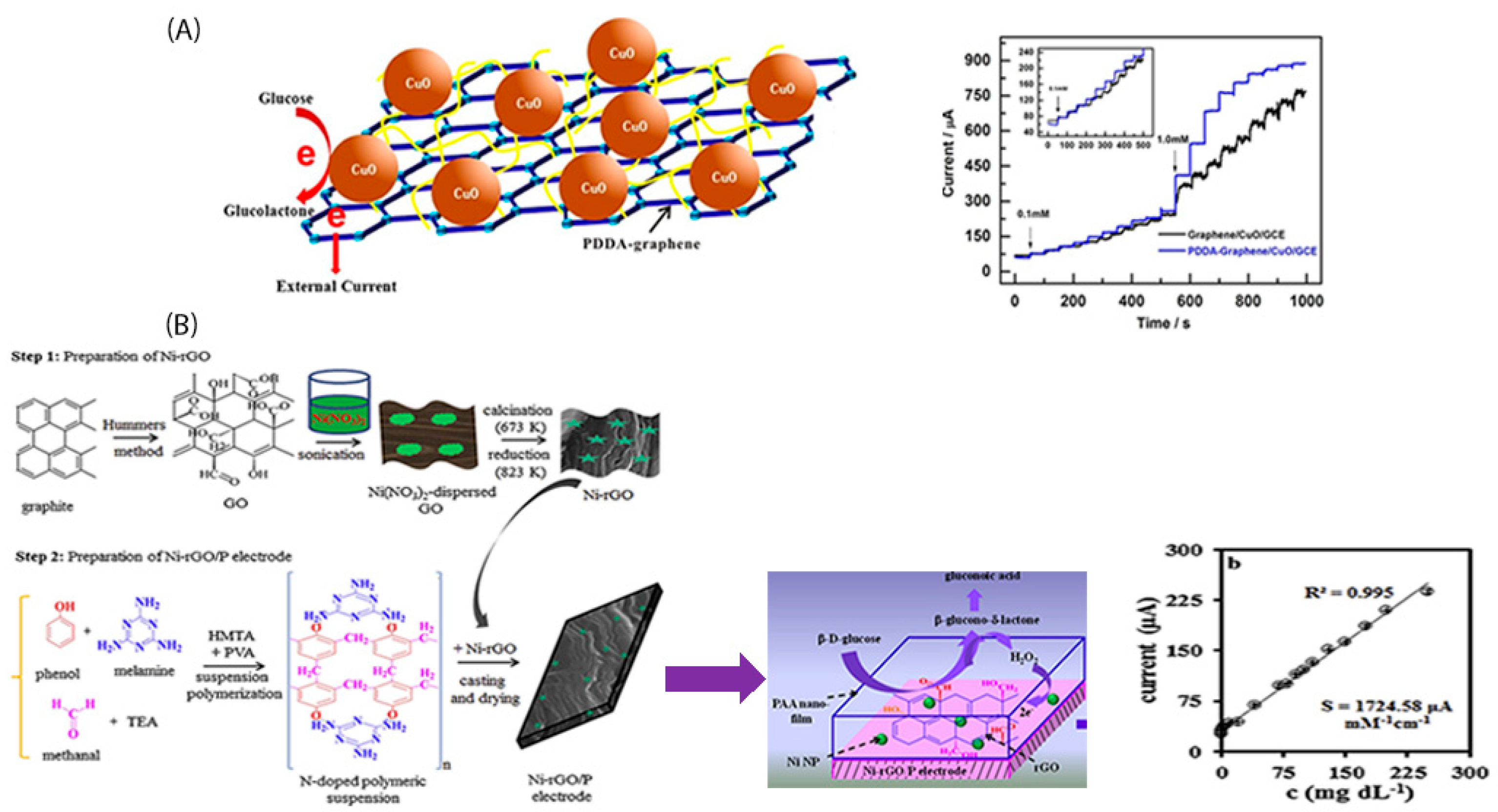
4. Polymer Functionalized Graphene-Based Electrodes
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/s22010355
References
- Singh, V.K.; Kumar, S.; Pandey, S.K.; Srivastava, S.; Mishra, M.; Gupta, G.; Malhotra, B.; Tiwari, R.; Srivastava, A. Fabrication of sensitive bioelectrode based on atomically thin CVD grown graphene for cancer biomarker detection. Biosens. Bioelectron. 2018, 105, 173–181.
- Wang, S.; Zhao, L.; Xu, R.; Ma, Y.; Ma, L. Facile fabrication of biosensors based on Cu nanoparticles modified as-grown CVD graphene for non-enzymatic glucose sensing. J. Electroanal. Chem. 2019, 853, 113527.
- Soganci, T.; Ayranci, R.; Harputlu, E.; Ocakoglu, K.; Acet, M.; Farle, M.; Unlu, C.G.; Ak, M. An effective non-enzymatic biosensor platform based on copper nanoparticles decorated by sputtering on CVD graphene. Sens. Actuators B Chem. 2018, 273, 1501–1507.
- Jiang, J.; Zhang, P.; Liu, Y.; Luo, H. A novel non-enzymatic glucose sensor based on a Cu-nanoparticle-modified graphene edge nanoelectrode. Anal. Methods-R. Soc. Chem. 2017, 9, 2205–2210.
- Ren, Z.; Mao, H.; Luo, H.; Liu, Y. Glucose sensor based on porous Ni by using a graphene bottom layer combined with a Ni middle layer. Carbon 2019, 149, 609–617.
- Usman, M.; Pan, L.; Farid, A.; Riaz, S.; Khan, A.S.; Peng, Z.Y.; Khan, M.A. Ultra-fast and highly sensitive enzyme-free glucose sensor based on 3D vertically aligned silver nanoplates on nickel foam-graphene substrate. J. Electroanal. Chem. 2019, 848, 113342.
- Jeong, H.; Kwac, L.K.; Hong, C.G.; Kim, H.G. Direct growth of flower like-structured CuFe oxide on graphene supported nickel foam as an effective sensor for glucose determination. Mater. Sci. Eng. C 2021, 118, 111510.
- Kurt Urhan, B.; Demir, Ü.; Öznülüer Özer, T.; Öztürk Doğan, H. Electrochemical fabrication of Ni nanoparticles-decorated electrochemically reduced graphene oxide composite electrode for non-enzymatic glucose detection. Thin Solid Films 2020, 693, 137695.
- Brouzgou, A.; Lo Vecchio, C.; Baglio, V.; Aricò, A.S.; Liang, Z.X.; Demin, A.; Tsiakaras, P. Glucose electrooxidation reaction in presence of dopamine and uric acid over ketjenblack carbon supported PdCo electrocatalyst. J. Electroanal. Chem. 2019, 855, 113610.
- Brouzgou, A.; Tsiakaras, P. Electrocatalysts for glucose electrooxidation reaction: A review. Top. Catal. 2015, 58, 1311–1327.
- Brouzgou, A.; Podias, A.; Tsiakaras, P. PEMFCs and AEMFCs directly fed with ethanol: A current status comparative review. J. Appl. Electrochem. 2013, 43, 119–136.
- Jiang, D.; Chu, Z.; Peng, J.; Luo, J.; Mao, Y.; Yang, P.; Jin, W. One-step synthesis of three-dimensional Co(OH)2/rGO nano-flowers as enzyme-mimic sensors for glucose detection. Electrochim. Acta 2018, 270, 147–155.
- Zhang, X.; Zhang, Z.; Liao, Q.; Liu, S.; Kang, Z.; Zhang, Y. Nonenzymatic glucose sensor based on in situ reduction of Ni/NiO-graphene nanocomposite. Sensors 2016, 16, 1791.
- Yang, H.; Bao, J.; Qi, Y.; Zhao, J.; Hu, Y.; Wu, W.; Wu, X.; Zhong, D.; Huo, D.; Hou, C. A disposable and sensitive non-enzymatic glucose sensor based on 3D graphene/Cu2O modified carbon paper electrode. Anal. Chim. Acta 2020, 1135, 12–19.
- Zhang, Y.; Xu, J.; Xia, J.; Zhang, F.; Wang, Z. MOF-Derived Porous Ni2P/Graphene Composites with Enhanced Electrochemical Properties for Sensitive Nonenzymatic Glucose Sensing. ACS Appl. Mater. Interfaces 2018, 10, 39151–39160.
- Liu, B.; Wang, X.; Liu, H.; Zhai, Y.; Li, L.; Wen, H. 2D MOF with electrochemical exfoliated graphene for nonenzymatic glucose sensing: Central metal sites and oxidation potentials. Anal. Chim. Acta 2020, 1122, 9–19.
- Chen, X.; Liu, D.; Cao, G.; Tang, Y.; Wu, C. In situ synthesis of a sandwich-like ZIF-67 heterostructure for highly sensitive nonenzymatic glucose sensing in human serums. ACS Appl. Mater. Interfaces 2019, 11, 9374–9384.
- Farid, M.M.; Goudini, L.; Piri, F.; Zamani, A.; Saadati, F. Molecular imprinting method for fabricating novel glucose sensor: Polyvinyl acetate electrode reinforced by MnO2/CuO loaded on graphene oxide nanoparticles. Food Chem. 2016, 194, 61–67.
- Mao, W.; He, H.; Ye, Z.; Huang, J. Three-dimensional graphene foam integrated with Ni(OH)2 nanosheets as a hierarchical structure for non-enzymatic glucose sensing. J. Electroanal. Chem. 2019, 832, 275–283.
- Cui, N.; Guo, P.; Yuan, Q.; Ye, C.; Yang, M.; Yang, M.; Chee, K.W.A.; Wang, F.; Fu, L.; Wei, Q.; et al. Single-Step Formation of Ni Nanoparticle-Modified Graphene–Diamond Hybrid Electrodes for Electrochemical Glucose Detection. Sensors 2019, 19, 2979.
- Karimi, M.; Mehrabadi, Z.; Farsadrooh, M.; Bafkary, R.; Derikvandi, H.; Hayati, P.; Mohammadi, K. Chapter 4—Metal–organic framework. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: London, UK, 2021; Volume 33, pp. 279–387.
- Sun, S.; Tang, Y.; Wu, C.; Wan, C. Phytic acid functionalized ZIF-67 decorated graphene nanosheets with remarkably boosted electrochemical sensing performance. Anal. Chim. Acta 2020, 1107, 55–62.
- Hao, J.; Li, C.; Wu, C.; Wu, K. In-situ synthesis of carbon-encapsulated Ni nanoparticles decorated graphene nanosheets with high reactivity toward glucose oxidation and sensing. Carbon 2019, 148, 44–51.
- Huang, L.; Su, J.; Song, Y.; Ye, R. Laser-Induced Graphene: En Route to Smart Sensing. Nano-Micro Lett. 2020, 12, 157.
- Rodriguez, R.D.; Khalelov, A.; Postnikov, P.S.; Lipovka, A.; Dorozhko, E.; Amin, I.; Murastov, G.V.; Chen, J.-J.; Sheng, W.; Trusova, M.E. Beyond graphene oxide: Laser engineering functionalized graphene for flexible electronics. Mater. Horiz. 2020, 7, 1030–1041.
- Reghunath, R.; devi, K.; Singh, K.K. Recent advances in graphene based electrochemical glucose sensor. Nano-Struct. Nano-Objects 2021, 26, 100750.
- Yang, G.-h.; Bao, D.-d.; Liu, H.; Zhang, D.-q.; Wang, N.; Li, H.-t. Functionalization of Graphene and Applications of the Derivatives. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1129–1141.
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513.
- Qu, L.; Dai, L. Substrate-Enhanced Electroless Deposition of Metal Nanoparticles on Carbon Nanotubes. J. Am. Chem. Soc. 2005, 127, 10806–10807.
- Tehrani, F.; Bavarian, B. Facile and scalable disposable sensor based on laser engraved graphene for electrochemical detection of glucose. Sci. Rep. 2016, 6, 27975.
- Zhao, J.; Zheng, C.; Gao, J.; Gui, J.; Deng, L.; Wang, Y.; Xu, R. Co3O4 nanoparticles embedded in laser-induced graphene for a flexible and highly sensitive enzyme-free glucose biosensor. Sens. Actuators B Chem. 2021, 347, 130653.
- Yang, J.; Lin, Q.; Yin, W.; Jiang, T.; Zhao, D.; Jiang, L. A novel nonenzymatic glucose sensor based on functionalized PDDA-graphene/CuO nanocomposites. Sens. Actuators B Chem. 2017, 253, 1087–1095.
- Bairagi, P.K.; Verma, N. Electro-polymerized polyacrylamide nano film grown on a Ni-reduced graphene oxide-polymer composite: A highly selective non-enzymatic electrochemical recognition element for glucose. Sens. Actuators B Chem. 2019, 289, 216–225.
