Cancer-associated fibroblasts (CAFs) are key components of the pancreatic tumor microenvironment, maintaining the extracellular matrix, while also being involved in intricate crosstalk with cancer cells and infiltrating immunocytes. Therefore, they are potential targets for developing therapeutic strategies against pancreatic ductal adenocarcinoma (PDAC). However, studies have demonstrated significant heterogeneity in CAFs with respect to their origins, spatial distribution, and functional phenotypes within the PDAC tumor microenvironment. Therefore, it is imperative to understand and delineate this heterogeneity prior to targeting CAFs for PDAC therapy.
- pancreatic ductal adenocarcinoma
- pancreatic cancer
- cancer-associated fibroblasts
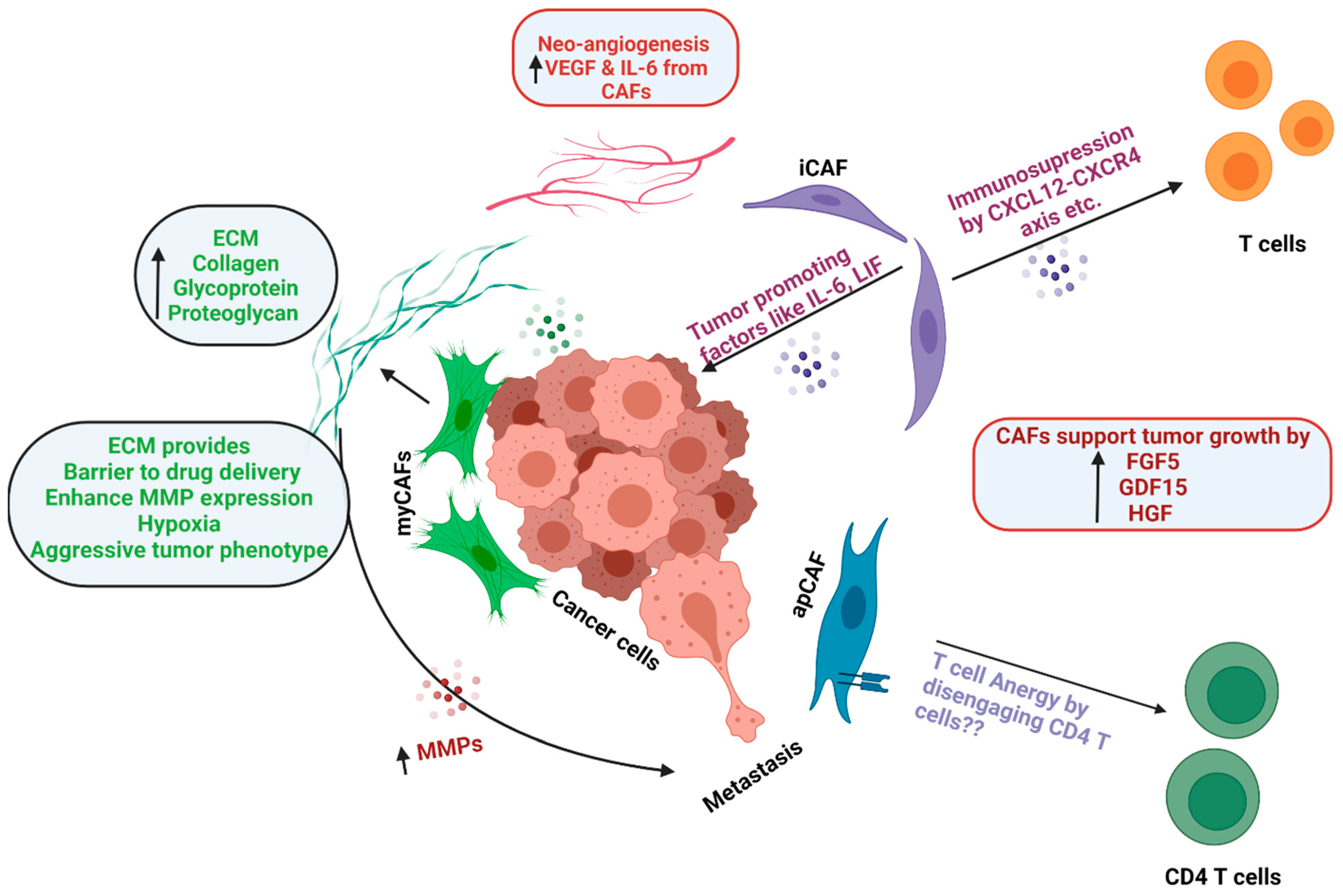
- myCAF
- iCAF
- apCAF
- CAF heterogeneity
1. Introduction
1.1 Cancer-Associated Fibroblasts (CAFs) - Origin and Heterogeneity
1.2 Functions of CAFs
2. CAFs as Therapeutic Targets
| S. No. | Target | Name of Therapeutic | Rationale Based on Pre-Clinical Studies | Current Status | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| 1. | Hedgehog Pathway | IPI-926 | Inhibition of the Hedgehog Pathway, leading to reduced CAF activation |
Phase II was halted due to the early detection of a shorter median overall survival (OS) in the experimental arm, compared to the placebo arm. | NCT01130142 |
| 2. | Hedgehog Pathway | Vismodegib | The phase Ib/II randomized clinical trial, evaluating the addition of Vismodegib to gemcitabine, showed no treatment benefit for OS or progression free survival (PFS). |
NCT01195415 | |
| 3. | Hyaluronic acid | PEGPH20 | Depletion of stroma by PEGPH20, which may synergize with immunotherapy | Clinical trials failed to show any benefit. | NCT03634332 |
| 4. | Angiotensin receptor | Losartan | Attenuation of collagen and hyaluronan deposition by CAFs through inhibition of TGF-β signaling | Encouraging results in locally advanced PDAC | NCT03563248 |
| 5. | Lysyl oxidase like-2 (LOXL2) | Simtuzumab | Inhibition of matrix-remodeling enzyme Lysyl oxidase-like 2, an ECM remodeling enzyme | Study completed, and addition of Simtuzumab to gemcitabine did not improve clinical outcomes |
NCT01472198 |
| 6. | CXCL12-CXCR4 Axis | Olaptesed (NOX-A12) | Modulation of PDAC TME by reducing immunosuppressive factors, as CXCL12 secreted by iCAFs promotes tumorigenesis by reducing CD8 T cell infiltration | Clinical trial ongoing | NCT03168139 |
| 7. | CXCL12-CXCR4 Axis | BL-8040 CXCR4 Antagonist |
Clinical trial ongoing | NCT02826486 | |
| 8. | IL-6 | Siltuximab | Combination of IL-6 and PD-L1 blockade decreases tumor growth, improves survival, and leads to increased infiltration of effector CD8+ T cells | Clinical trial ongoing | NCT04191421 |
| 9. | Vitamin D receptor (VDR) | Paricalcitol (Vitamin D Receptor Agonist) | Modulating signaling in tumor microenvironment. CAFs highly express VDR, and treating them with Vitamin D can induce a quiescent phenotype |
Clinical trials ongoing | NCT03520790 |
| NCT03300921 | |||||
| NCT02754726 | |||||
| 10. | Vitamin D receptor (VDR) | Vitamin D3 | Clinical trials ongoing | NCT03472833 | |
| 11. | Stroma | All Trans Retinoic Acid (ATRA) | Inducing CAF quiescence and decreasing motility, leading to decreased tumor growth through decreased Wnt-β Catenin signaling | Clinical trials ongoing | NCT03307148 |
| 12. | IL-1R | Anakinra (IL-1R antagonist) |
By switching iCAF to a myCAF phenotype | Clinical trials ongoing | NCT02550327 |
CXC chemokine ligand 12 (CXCL12).
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms222413408
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921.
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220.
- Luo, G.; Fan, Z.; Gong, Y.; Jin, K.; Yang, C.; Cheng, H.; Huang, D.; Ni, Q.; Liu, C.; Yu, X. Characteristics and Outcomes of Pancreatic Cancer by Histological Subtypes. Pancreas 2019, 48, 817–822.
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022.
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020.
- Jain, T.; Dudeja, V. The war against pancreatic cancer in 2020—advances on all fronts. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 99–100.
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540.
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835.
- Mishra, P.J.; Mishra, P.J.; Humeniuk, R.; Medina, D.J.; Alexe, G.; Mesirov, J.P.; Ganesan, S.; Glod, J.W.; Banerjee, D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008, 68, 4331–4339.
- Ishii, G.; Sangai, T.; Oda, T.; Aoyagi, Y.; Hasebe, T.; Kanomata, N.; Endoh, Y.; Okumura, C.; Okuhara, Y.; Magae, J.; et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem. Biophys. Res. Commun. 2003, 309, 232–240.
- Okumura, T.; Ohuchida, K.; Kibe, S.; Iwamoto, C.; Ando, Y.; Takesue, S.; Nakayama, H.; Abe, T.; Endo, S.; Koikawa, K.; et al. Adipose tissue-derived stromal cells are sources of cancer-associated fibroblasts and enhance tumor progression by dense collagen matrix. Int. J. Cancer 2019, 144, 1401–1413.
- Zeltz, C.; Primac, I.; Erusappan, P.; Alam, J.; Noel, A.; Gullberg, D. Cancer-associated fibroblasts in desmoplastic tumors: Emerging role of integrins. Semin. Cancer Biol. 2020, 62, 166–181.
- DuFort, C.C.; DelGiorno, K.E.; Hingorani, S.R. Mounting Pressure in the Microenvironment: Fluids, Solids, and Cells in Pancreatic Ductal Adenocarcinoma. Gastroenterology 2016, 150, 1545–1557 e1542.
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254.
- Wang, Z.; Liu, J.; Huang, H.; Ye, M.; Li, X.; Wu, R.; Liu, H.; Song, Y. Metastasis-associated fibroblasts: An emerging target for metastatic cancer. Biomark. Res. 2021, 9, 47.
- Fukumura, D.; Xavier, R.; Sugiura, T.; Chen, Y.; Park, E.C.; Lu, N.; Selig, M.; Nielsen, G.; Taksir, T.; Jain, R.K.; et al. Tumor induction of VEGF promoter activity in stromal cells. Cell 1998, 94, 715–725.
- Nagasaki, T.; Hara, M.; Nakanishi, H.; Takahashi, H.; Sato, M.; Takeyama, H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: Anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br. J. Cancer 2014, 110, 469–478.
- Zhang, Y.; Recouvreux, M.V.; Jung, M.; Galenkamp, K.M.O.; Li, Y.; Zagnitko, O.; Scott, D.A.; Lowy, A.M.; Commisso, C. Macropinocytosis in Cancer-Associated Fibroblasts is Dependent on CaMKK2/ARHGEF2 Signaling and Functions to Support Tumor and Stromal Cell Fitness. Cancer Discov. 2021, 11.
- Shi, Y.; Gao, W.; Lytle, N.K.; Huang, P.; Yuan, X.; Dann, A.M.; Ridinger-Saison, M.; DelGiorno, K.E.; Antal, C.E.; Liang, G.; et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 2019, 569, 131–135.
- Tape, C.J.; Ling, S.; Dimitriadi, M.; McMahon, K.M.; Worboys, J.D.; Leong, H.S.; Norrie, I.C.; Miller, C.J.; Poulogiannis, G.; Lauffenburger, D.A.; et al. Oncogenic KRAS Regulates Tumor Cell Signaling via Stromal Reciprocation. Cell 2016, 165, 1818.
- Bruzzese, F.; Hagglof, C.; Leone, A.; Sjoberg, E.; Roca, M.S.; Kiflemariam, S.; Sjoblom, T.; Hammarsten, P.; Egevad, L.; Bergh, A.; et al. Local and systemic protumorigenic effects of cancer-associated fibroblast-derived GDF15. Cancer Res. 2014, 74, 3408–3417.
- Fearon, D.T. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol. Res. 2014, 2, 187–193.
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012, 122, 899–910.
