Edible films and coatings allow preserving fresh and processed food, maintaining quality, preventing microbial contamination and/or oxidation reactions and increasing the shelf life of food products. The structural matrix of edible films and coatings is mainly constituted by proteins, lipids or polysaccharides. However, it is possible to increase the bioactive potential of these polymeric matrices by adding phenolic compounds obtained from plant extracts. Phenolic compounds are known to possess several biological properties such as antioxidant and antimicrobial properties. Incorporating phenolic compounds enriched plant extracts in edible films and coatings contribute to preventing food spoilage/deterioration and the extension of shelf life.
- edible coatings and films
- phenolic compounds
- starches
1. Introduction
Biopolymers are organic polymers and according with its origin are classified, mainly, into three types: biomass-derived polymers (e.g., proteins and polysaccharides including chitosan, starch and cellulose); synthetic polymers derived from oil or biomass monomers such as polylactic acid (PLA), polycaprolactones (PCL) and polyvinyl alcohol (PVA); polymers produced by natural microorganisms or genetically modified (e.g., polyhydroxyalcanoates and bacterial cellulose) [1][2][3][4][5]. In general, bioplastics present some advantages relative to common plastics: namely, they use less energy and are biodegradable, safe and emit less greenhouse gases; provide longer shelf life and are suitable for the production of compost; the chemical structure is more diversified, making possible the customization of the properties of the final package according to the type of food; and possibility of nanoparticles incorporation and adding or improving important properties such as thermic stability, gas impermeability/barrier including to oxygen, antimicrobial properties and strength [6][4]. Regardless of these advantages, bioplastics have some issues concerning its mechanical and barriers properties, which require the addition of additives or other synthetic polymers (e.g., via hydroxypropylation) improving the referred properties [1][7]. Therefore, biopolymers may not be 100% made of renewable sources as some are blends of natural polymers with synthetic polymers or incorporate additives that improve the functional properties of the final package [1].
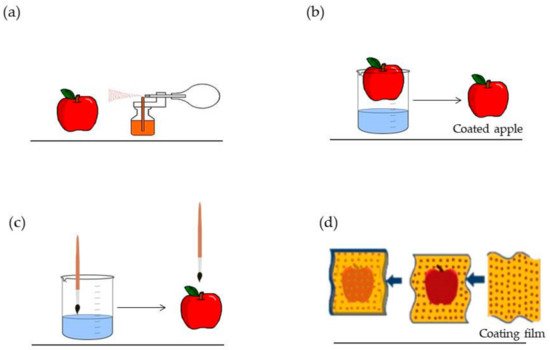
Biopolymers are used to prepare edible films and coatings, materials with thickness below 0.3 mm that are produced through a blend of biopolymers with different additives dispersed in an aqueous phase, and are safe to eat [8][9][10]. Edible films and coatings are easily manufactured, economically viable [11] and provide mechanical protection and improvement of food quality through moisture regulation and internal equilibrium between gases and solutes [8][10][11]. In addition to that, these materials can protect against UV radiation, fungi and bacterial contamination [8]. Edible films and coatings have the capacity to carry bioactive compounds, such as phenolic compounds, vitamins, nutraceuticals and probiotics, which improve food quality and, at the same time, provide additional health properties to the consumer after food consumption [8][12]. All these characteristics contribute to shelf-life increase [8][10] and a reduction in the use of additives [13]. In general, edible films and coatings are classified into the following: oil/water emulsions and colloidal dispersions [8]. They can be applied by dipping, spreading, spraying and wrapping (Figure 1) [8][10][11]. They can also be classified according to the type of application: edible coatings applied by dipping, spreading or spraying and edible films produced by compression molding, solvent casting or extrusion and that are used to wrap food [8][10].
2. Gums Used in Food Packaging
| Polysaccharide | Food Application | Bioactive Compounds Incorporated | Type of Packaging | Application Coating Method | Results | References |
|---|---|---|---|---|---|---|
| Xanthan | Melon | β-carotene nanoparticles | Coating | Immersion | Improvement of coatings properties and increase in shelf time to 21 days at 4 °C | [25][26] |
| Refrigerated fish | Chitosan | Film | - | Inhibition of the growth of Staphylococcus coagulase-positive, Salmonella spp. and coliforms at 45 °C Quality preservation |
[27] | |
| Acerola | - | Coating | - | Reduction of weight loss and the respiration process Increase in shelf life (prolongation of 6 days at 30 °C without deterioration signs |
[5] | |
| Pear | - | Coating | - | Retained the weight during 9 days of storage Prevention of oxidation |
[28] | |
| Baby carrots | α-tocopherol | Coating | Dipping | Edible coatings improve the surface colour without organoleptic properties alterations | [29] | |
| Galactomannan | Ricotta cheese | Nisin | Coating | Dipping | Delay of microbial growth during 28 days Weight loss and moisture content decreasing |
[30][31] |
| Guar gum | Fruits | Nisin | - | - | Decrease in gas transfer rates | [31][32][33] |
| - | Ag/Cu nanoparticles | Film | Spreading | Strong antibacterial activity against Gram-positive Listeria monocytogenes bacteria and Gram-negative Salmonella enterica sv typhimurium Excellent UV, light and oxygen barrier capability |
- | |
| Roma tomato | - | Coating | Immersion | Firmness enhancement Reduce the weight loss Retarded loss of total acidity Respiration rate decrease |
[33] | |
| Blackberries | - | - | - | Shelf-life extension for 13 days | [34] | |
| lemon | Spice extracts | Coating | Dipping | Shelf-life extension Maintenance of quality during cold storage Inhibition of bacterial growth |
[35] | |
| Litchi fruits | - | Coating | - | Maintenance of fruit quality Shelf-life extension up to 10 days under low temperature storage |
[36] | |
| Red chilli pepper | - | Coating | Dipping | Maintenance of fruit quality without deterioration during 20 days at storage temperature of 6 °C | [37] | |
| Arabic gum | Tomato | - | Coating | Immersion | Delay of the ripening process Shelf-life extension for 20 days without deterioration and off-flavours, stored at 20 °C |
[38] |
| Guava | Sodium caseinate and Tulsi extract | Coating | Dipping | Maintenance of suitable internal gas composition delaying ripening Shelf-life of 7 days at 28 ± 2 °C compared to 4 days of control |
[39] | |
| Green chillies | Glycerol Thyme oil Tween 80 |
Coating | Dipping | Preservation of the quality and organoleptic properties During 12 days |
[40] | |
| Persimmon fruits | - | Coating | Dipping | Lower weight loss, membrane leakage, H2O2 and malondialdehyde content relative to the control Suppression of the increase in activities of polygalacturonase, pectin methylesterase and cellulase enzymes Higher superoxide dismutase, peroxidase, ascorbate peroxidase and catalase activities |
[41] | |
| Mangoes | - | Coating | Dipping | Gas and water vapour barrier properties Slower the ripening process Shelf-life extension for 15 days relative to less of 10 days in control |
[42] | |
| Gum tragacanth | Button mushroom | Aloe vera leaves extract | Coating | Immersion | Slow the loss of weight and colour changes under cold storage | [43] |
| Fresh apricots | Chitosan | Coating | Dipping | Improvement of firmness and stability in terms of weight loss, pH and moisture content during storage | [44] | |
| - | Coating | Dipping | Good sensorial qualities Antioxidant properties Maintenance of ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD) and peroxidase (POD) enzymes activities Inhibition of polygalacturonase (PG), pectin methylesterase (PME) and cellulase (CX) enzymes activities |
[45] | ||
| Locust bean | Fortune mandarins | - | Coating | Immersion | Weight loss delay Improve gloss of the fruits |
[46] |
| Konjac glucomanann | Fresh-cut cucumber | Saffron petal extract | Film | - | Reduction in the water vapour permeability Antimicrobial properties against Escherichia coli (E. coli), Shigella sonnei, Salmonella Typhi, Staphylococcus aureus (S. aureus) and Bacillus cereus Preservation of fruits and vegetables quality Shelf-life extension |
[47] |
| Guava | - | Coating | Immersion | Maintenance of firmness and colour Reduce weight loss |
[48] | |
| Cantaloupe | Potassium sorbate | Coating | - | Maintenance of weight loss, hardness and firmness Inhibition of microbial growth Preservation of sliced cantaloupe up to 5 days |
[49] | |
| Gellan gum | Mango | - | Coating | Dipping | Improvement of sensorial characteristics namely appearance and firmness Stabilization of colour and volatiles composition during storage |
[50] |
| Almond gum/Persian gum | Tomato | - | Coating | Immersion | Delay changes in colour, weight loss, firmness, acidity, ascorbic acid content, soluble solids concentration and decay percentage during a storage period of 20 days. | [51] |
| Cherries | Gum Arabic | Coating | Immersion | Delay the ripening process and increase the shelf life of cherries without spoilage or off-flavour | [52][53] |
3. Starches Used in Food Packaging
| Starch Source | Food Application | Bioactive Compounds | Type of Packaging | Application Coating Method | Results | References |
|---|---|---|---|---|---|---|
| Tropical fruits: banana “Pear”, soursop, stenospermocarpic mango | Stenospermocarpic mangoes | - | Coating | Immersion | Mango starch showed better results than the other starches coatings tested: less weight loss, greater firmness, high content of total soluble solid and shelf-life extension up to 15 days (10 days at 10 °C and 5 days at 22 °C) | [61] |
| Corn | Banana | Gum Arabic Glycerol Sorbitol |
Film | Dipping | At a temperature of 26 °C, the coated fruits lose less weight than control (uncoated fruits), retaining firmness and delaying the ripening process | [62] |
| Cassava | Mango | Chitosan | Coating | Immersion | At 25 °C, the coating showed good sensorial qualities and decreased respiration rate without prejudicing the ripening process Shelf-life extension up to 3 days |
[63] |
| Rice and cassava | Pummelo | Pummelo juice | Film | Immersion | Lower weight loss Physical appearance stabilization |
[64] |
| Potato | “Orri” mandarins | Glycerol | Coating | Immersion | Weight loss reduction | [65] |
| Tapioca | Cauliflower | Gelatin | Coating | Dipping | Weight loss reduction Total soluble solids increase |
[66] |
| Cassava | Blackberries | Chitosan Glycerol |
Coating | Immersion | Good sensorial properties (colour maintenance) Weight loss reduction and firmness increase during 10 days of storage |
[67] |
| Banana “Pear” | Mango | Pectin | Coating | - | High firmness High total soluble solids Post harvested period extended to 21 days Retention of colour mango fruits |
[68] |
| Wheat | Plums | - | Coating | Immersion | No colour changes Improvement of water vapour and oxygen permeability |
[69] |
| Chickpea | Papaya | Glycerol Stearic acid |
Film | Dipping | Reduced weight loss, better firmness and colour retention at 10 °C during 10 days of storage | [70] |
| Cassava | Black mulberry | Chitosan | Coating | Immersion | Minimized weight loss and mold decay during cold storage (5 °C) for 16 days No alterations at firmness, colour and anthocyanin content |
[71] |
| Tomato | Vegetable oil, glycerol, soy lecithin and cellulose and derivates | Film | Immersion | Delay changes in firmness, weight, titratable acidity, pH, total soluble solids, sugar/acidity ratio and colour development Increased shelf life of tomatoes stored at 20 ± 2 °C up to 1 month |
[72] | |
| Toasted groundnuts |
Soy protein concentrate | Coating | Dipping | Excellent sensorial properties Shelf-life extension during 14 days at ambient temperature |
[73] | |
| Pineapple | Alginate Ascorbic acid Glycerol |
Coating | Immersion | Lower levels of reducing and total sugars Better appearance and general acceptance during room storage for 18 days Preservation of sweetness, taste and odour and better appearance |
[74] | |
| Guavas | Gelatin Chitosan |
Film | Immersion | Decrease weight loss Shelf life increased up to 9 days relative to non-coated guavas Slowed the ripening process (after 27 days of storage, the fruits had not reached senescence) |
[75] | |
| Waxy corn | Rice cakes | Gellan gum | Film | Dipping | Preservation of texture of rice cakes during 24 h of storage Reduction in moisture loss and delay of hardening |
[76] |
| Potato | Grape | Rice bran oil | Coating | - | Good water vapour barrier Preservation of the grape’s quality Long-term shelf-life extension |
[77] |
| Corn | Apples | Papaya polysaccharides | Film | - | Increase in swelling and tensile strength, reduction in thickness, transparency and solubility Improved sensorial acceptance |
[78] |
4. Methods of Incorporation Phenolic Compounds in Gums and Starch-Based Coatings and Films
5. Phenolic Compounds Incorporated in Gums and Starches for Preparing Active Films
6. Intelligent and Active Starch/Gums Films with Phenolic Compounds
7. Conclusions
8. Future Perspectives
This entry is adapted from the peer-reviewed paper 10.3390/coatings11111393
References
- Adeyeye, O.A.; Sadiku, E.R.; Babu Reddy, A.; Ndamase, A.S.; Makgatho, G.; Sellamuthu, P.S.; Perumal, A.B.; Nambiar, R.B.; Fasiku, V.O.; Ibrahim, I.D.; et al. The Use of Biopolymers in Food Packaging. In Green Biopolymers and Their Nanocomposites. Materials Horizons: From Nature to Nanomaterials; Gnanasekaran, D., Ed.; Springer: Singapore, 2019; pp. 137–158.
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380.
- Sahu, M.; Sahoo, P.K. Bio polymers: Sustainable alternative for food packaging. Int. J. Eng. Manag. Res. 2017, 28–32.
- Grujić, R.; Vujadinović, D.; Savanović, D. Biopolymers as food packaging materials. Adv. Appl. Ind. Biomater. 2017, 139–160.
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-based membranes in food packaging applications. Membranes 2016, 6, 22.
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625.
- Samsudin, H.; Hani, N.M. Use of starch in food packaging. Starch-Based Mater. Food Packag. Process. Charact. Appl. 2017, 229–256.
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249.
- Shendurse, A. Milk protein based edible films and coatings–preparation, properties and food applications. J. Nutr. Health Food Eng. 2018, 8, 219–226.
- Tavassoli-Kafrani, E.; Gamage, M.V.; Dumée, L.F.; Kong, L.; Zhao, S. Edible films and coatings for shelf life extension of mango: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–26.
- Sharma, H.P.; Chaudhary, V.; Kumar, M. Importance of edible coating on fruits and vegetables: A review. J. Pharmacogn. Phytochem. 2019, 8, 4104–4110.
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive edible films for food applications: Mechanisms of antimicrobial and antioxidant activity. Crit. Rev. Food Sci. Nutr. 2018, 59, 3431–3455.
- Han, J.H. Edible films and coatings: A review. Innov. Food Packag. 2014, 213–255.
- Lazaridou, A.; Biliaderis, C.G. Edible films and coatings with pectin. Pectin Technol. Physiol. Prop. 2020, 99–123.
- Pandey, H.; Kumar, S. Butylated hydroxytoluene and Butylated hydroxyanisole induced cyto-genotoxicity in root cells of Allium cepa L. Heliyon 2021, 7, e07055.
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674.
- Pop, O.L.; Pop, C.R.; Dufrechou, M.; Vodnar, D.C.; Socaci, S.A.; Dulf, F.V.; Minervini, F.; Suharoschi, R. Edible films and coatings functionalization by probiotic incorporation: A review. Polymers 2019, 12, 12.
- Alizadeh-Sani, M.; Ehsani, A.; Moghaddas Kia, E.; Khezerlou, A. Microbial gums: Introducing a novel functional component of edible coatings and packaging. Appl. Microbiol. Biotechnol. 2019, 103, 6853–6866.
- da Silva, D.A.; Aires, G.C.M.; Pena, R.D.S. Gums—Characteristics and Applications in the Food Industry. In Innovation in the Food Sector through the Valorization of Food and Agro-Food By-Products; IntechOpen: Rijeka, Croatia, 2020.
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973.
- Katzbauer, B. Properties and applications of xanthan gum. Polym. Degrad. Stab. 1998, 59, 81–84.
- Yousuf, B.; Wu, S.; Gao, Y. Characteristics of karaya gum based films: Amelioration by inclusion of schisandra chinensis oil and its oleogel in the film formulation. Food Chem. 2021, 345, 128859.
- Khezerlou, A.; Zolfaghari, H.; Banihashemi, S.A.; Forghani, S.; Ehsani, A. Plant gums as the functional compounds for edible films and coatings in the food industry: A review. Polym. Adv. Technol. 2021, 32, 2306–2326.
- de Boer, F.Y.; Imhof, A.; Velikov, K.P. Encapsulation of colorants by natural polymers for food applications. Color. Technol. 2019, 135, 183–194.
- Salehi, F. Edible coating of fruits and vegetables using natural gums: A review. Int. J. Fruit Sci. 2020, 20, S570–S589.
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, J.F. The release kinetics of β-carotene nanocapsules/xanthan gum coating and quality changes in fresh-cut melon (cantaloupe). Carbohydr. Polym. 2017, 157, 1874–1882.
- Lima, M.D.M.; Carneiro, L.C.; Machado, M.R.G.; Dias, A.R.G.; Zavareze, E.D.R.; Prentice, C.; Moreira, A.D.S. Application of films based on chitosan and xanthan gum in refrigerated fish conservation. Brazilian Arch. Biol. Technol. 2020, 63, 10.
- Mohamed, A.; Aboul-Anean, H.; Hassan, M. Utilization of edible coating in extending the shelf life of minimally processed prickly pear. J. Appl. Sci. Res. 2013, 9, 1202–1208.
- Mei, Y.; Zhao, Y.; Yang, J.; Furr, H.C. Using edible coating to enhance nutritional and sensory qualities of baby carrots. J. Food Sci. 2002, 67, 1964–1968.
- Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Galactomannans use in the development of edible films/coatings for food applications. Trends Food Sci. Technol. 2011, 22, 662–671.
- Martins, J.T.; Cerqueira, M.A.; Souza, B.W.S.; Avides, M.D.C.; Vicente, A.A. Shelf life extension of ricotta cheese using coatings of galactomannans from nonconventional sources incorporating nisin against listeria monocytogenes. J. Agric. Food Chem. 2010, 58, 1884–1891.
- Arfat, Y.A.; Ejaz, M.; Jacob, H.; Ahmed, J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohydr. Polym. 2017, 157, 65–71.
- Ruelas-Chacon, X.; Contreras-Esquivel, J.C.; Montañez, J.; Aguilera-Carbo, A.F.; Reyes-Vega, M.L.; Peralta-Rodriguez, R.D.; Sanchéz-Brambila, G. Guar gum as an edible coating for enhancing shelf-life and improving postharvest quality of roma tomato (Solanum lycopersicum L.). J. Food Qual. 2017, 2017, 8608304.
- Pérez, D.A.; Gómez, J.M.; Castellanos, D.A. Combined modified atmosphere packaging and guar gum edible coatings to preserve blackberry (Rubus glaucus Benth). Food Sci. Technol. Int. 2020, 27, 353–365.
- Naeem, A.; Abbas, T.; Ali, T.M.; Hasnain, A. Application of guar gum-based edible coatings supplemented with spice extracts to extend post-harvest shelf life of lemon (Citrus limon). Qual. Assur. Saf. Crop. Foods 2019, 11, 241–250.
- Kumar, R.; Dongariyal, A.; Rai, R.; Kumar Arya, M.; Mishra, N. Effect of edible coating and packaging on postharvest life and quality of litchi (Litchi chinensis Sonn.) fruits during storage. J. Pharmacogn. Phytochem. 2020, 9, 3018–3023.
- Minh, N.P.; Pham, V.T.; Van Tuan, T.; Tuyen, T.T.; Mai, D.K. Application of guar gum as edible coating to prolong shelf life of red chilli pepper (Capsicum frutescens L.) fruit during preservation—ProQuest. J. Pharm. Sci. Res. 2019, 11, 1474–1478.
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P.G. Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2010, 58, 42–47.
- Murmu, S.B.; Mishra, H.N. Optimization of the arabic gum based edible coating formulations with sodium caseinate and tulsi extract for guava. LWT 2017, 80, 271–279.
- Valiathan, S.; Athmaselvi, K.A. Gum arabic based composite edible coating on green chillies. Int. Agrophys. 2018, 32, 193–202.
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ali, S.; Canan, İ. Postharvest application of gum arabic edible coating delays ripening and maintains quality of persimmon fruits during storage. J. Food Process. Preserv. 2020, 44, e14583.
- LL, D.; JM, N.; WM, J.; SM, R. Effect of edible gum Arabic coating on the shelf life and quality of mangoes (Mangifera indica) during storage. J. Food Sci. Technol. 2020, 57, 79–85.
- Mohebbi, M.; Ansarifar, E.; Hasanpour, N.; Amiryousefi, M.R. Suitability of aloe vera and gum tragacanth as edible coatings for extending the shelf life of button mushroom. Food Bioprocess Technol. 2011, 5, 3193–3202.
- Ziaolhagh, S.H.; Kanani, S. Extending the shelf life of apricots by using gum tragacanth-chitosan edible coating. J. Agr. Sci. Tech 2021, 23, 319–331.
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Sardar, H.; Saddiq, B. Tragacanth gum coating modulates oxidative stress and maintains quality of harvested apricot fruits. Int. J. Biol. Macromol. 2020, 163, 2439–2447.
- Rojas-Argudo, C.; del Río, M.A.; Pérez-Gago, M.B. Development and optimization of locust bean gum (LBG)-based edible coatings for postharvest storage of “Fortune” mandarins. Postharvest Biol. Technol. 2009, 52, 227–234.
- Hashemi, S.M.B.; Jafarpour, D. The efficacy of edible film from Konjac glucomannan and saffron petal extract to improve shelf life of fresh-cut cucumber. Food Sci. Nutr. 2020, 8, 3128.
- Barion, G.C.; Vital, A.C.P.; Matumoto-Pintro, P.T.; Rosa, C.I.L.F. Influence of glucomannan edible coating in guava quality during storage. Res. Soc. Dev. 2020, 9, e2639108432.
- Morakot, N.; Vatanyoopaisarn, S.; Thumthanaruk, B.; Wongsa, J.; Puttanlek, C.; Uttapap, D.; Lamsal, B.P.; Rungsardthong, V. Effect of glucomannan and potassium sorbate on quality and shelf life of fresh-cut cantaloupe. Sci. Eng. Health Stud. 2020, 14, 123–131.
- Danalache, F.; Carvalho, C.Y.; Alves, V.D.; Moldão-Martins, M.; Mata, P. Optimisation of gellan gum edible coating for ready-to-eat mango (Mangifera indica L.) bars. Int. J. Biol. Macromol. 2016, 84, 43–53.
- Mahfoudhi, N.; Chouaibi, M.; Hamdi, S. Effectiveness of almond gum trees exudate as a novel edible coating for improving postharvest quality of tomato (Solanum lycopersicum L.) fruits. Food Sci. Technol. Int. 2014, 20, 33–43.
- Galus, S.; Kibar, E.A.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674.
- Mahfoudhi, N.; Hamdi, S. Use of almond gum and gum arabic as novel edible coating to delay postharvest ripening and to maintain sweet cherry (Prunus avium) quality during storage. J. Food Process. Preserv. 2015, 39, 1499–1508.
- Lauer, M.K.; Smith, R.C. Recent advances in starch-based films toward food packaging applications: Physicochemical, mechanical, and functional properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083.
- Sapper, M.; Chiralt, A. Starch-based coatings for preservation of fruits and vegetables. Coatings 2018, 8, 152.
- Sanyang, M.L.; Ilyas, R.A.; Sapuan, S.M.; Jumaidin, R. Sugar palm starch-based composites for packaging applications. In Bionanocomposites for Packaging Applications; Springer: Cham, Switzerland, 2017; pp. 125–147.
- Mironescu, M.; Lazea-Stoyanova, A.; Barbinta-Patrascu, M.E.; Virchea, L.-I.; Rexhepi, D.; Mathe, E.; Georgescu, C. Green design of novel starch-based packaging materials sustaining human and environmental health. Polymers 2021, 13, 1190.
- Hatmi, R.U.; Apriyati, E.; Cahyaningrum, N. Edible coating quality with three types of starch and sorbitol plasticizer. E3S Web Conf. EDP Sci. 2020, 142, 02003.
- Sadeghizadeh-Yazdi, J.; Habibi, M.; Kamali, A.A.; Banaei, M. Application of edible and biodegradable starch-based films in food packaging: A systematic review and meta-analysis. Curr. Res. Nutr. Food Sci. 2019, 7, 624–637.
- Liu, Z. Edible films and coatings from starches. Innov. Food Packag. 2005, 318–337.
- Hernández-Guerrero, S.E.; Balois-Morales, R.; Palomino-Hermosillo, Y.A.; López-Guzmán, G.G.; Berumen-Varela, G.; Bautista-Rosales, P.U.; Alejo-Santiago, G. Novel edible coating of starch-based stenospermocarpic mango prolongs the shelf life of mango “ataulfo” fruit. J. Food Qual. 2020, 2020, 1320357.
- Razak, A.S.; Lazim, A.M. Starch-based edible film with gum arabic for fruits coating. AIP Conf. Proc. 2015, 1678, 050020.
- Camatari, F.O.D.S.; Santana, L.C.L.D.A.; Carnelossi, M.A.G.; Alexandre, A.P.S.; Nunes, M.L.; Goulart, M.O.F.; Narain, N.; Silva, M.A.A.P.D. Impact of edible coatings based on cassava starch and chitosan on the post-harvest shelf life of mango (Mangifera indica) ‘Tommy Atkins’ fruits. Food Sci. Technol. 2017, 38, 86–95.
- Kerdchoechuen, O.; Laohakunjit, N.; Tussavil, P.; Kaisangsri, N.; Matta, F.B. Effect of starch-based edible coatings on quality of minimally processed pummelo (Citrus maxima merr.). Int. J. Fruit Sci. 2011, 11, 410–423.
- Soto-Muñoz, L.; Palou, L.; Argente-Sanchis, M.; Ramos-López, M.A.; Pérez-Gago, M.B. Optimization of antifungal edible pregelatinized potato starch-based coating formulations by response surface methodology to extend postharvest life of ‘Orri’ mandarins. bioRxiv 2020, 288, 110394.
- Kasim, R.; Kasim, M.U. The effect of tapioca-starch edible coating on quality of fresh-cut cauliflower during storage. In Proceedings of the 3rd International Symposium for Agriculture and Food—ISAF, Ohrid, North Macedonia, 18–20 October 2017.
- Cortés Rodríguez, M.; Villegas Yépez, C.; Gil González, J.H.; Ortega-Toro, R. Effect of a multifunctional edible coating based on cassava starch on the shelf life of Andean blackberry. Heliyon 2020, 6, e03974.
- Bello-Lara, J.E.; Balois-Morales, R.; Juárez-López, P.; Alia-Tejacal, I.; Peña-Valdivia, C.B.; Jiménez-Zurita, J.O.; Sumaya-Martínez, M.T.; Jiménez-Ruíz, E.I.; Bello-Lara, J.E.; Balois-Morales, R.; et al. Coatings based on starch and pectin from ‘Pear’ banana (Musa ABB), and chitosan applied to postharvest ‘Ataulfo’ mango fruit. Rev. Chapingo. Ser. Hortic. 2016, 22, 209–218.
- Basiak, E.; Geyer, M.; Debeaufort, F.; Lenart, A.; Linke, M. Relevance of interactions between starch-based coatings and plum fruit surfaces: A physical-chemical analysis. Int. J. Mol. Sci. 2019, 20, 2220.
- Kathiresan, S.; Lasekan, O. Effects of glycerol and stearic acid on the performance of chickpea starch-based coatings applied to fresh-cut papaya. CyTA-J. Food 2019, 17, 365–374.
- Ojeda, G.A.; Arias Gorman, A.M.; Sgroppo, S.C.; Zaritzky, N.E. Application of composite cassava starch/chitosan edible coating to extend the shelf life of black mulberries. J. Food Process. Preserv. 2021, 45, e15073.
- Adjouman, Y.D.; Nindjin, C.; Kouassi, K.N.; Tetchi, F.A.; Amani, G.G.; Sindic, M. Effect of edible coating based on improved cassava starch on post-harvest quality of fresh tomatoes (Solanum lycopersicum L.). Int. J. Nutr. Sci. Food Technol. 2018, 4, 1–10.
- Chinma, C.E.; Ariahu, C.C.; Abu, J.Q. Shelf life extension of toasted groundnuts through the application of cassava starch and soy protein-based edible coating. Niger. Food J. 2014, 32, 133–138.
- Henrique, G.; Guimarães, C.; Lima Dantas, R.; Bezerra De Sousa, A.S.; Soares, L.G.; De Sá Melo, R.; Sousa Da Silva, R.; Lima, R.P.; Nunes Mendonça, R.M.; Beaudry, R.M.; et al. African journal of agricultural research impact of cassava starch-alginate based coatings added with ascorbic acid and elicitor on quality and sensory attributes during pineapple storage. Afr. J. Agric. Res. 2017, 12, 664–673.
- Silva, O.A.; Pellá, M.C.G.; Friedrich, J.C.C.; Pellá, M.G.; Beneton, A.G.; Faria, M.G.I.; Colauto, G.A.L.; Caetano, J.; Simões, M.R.; Dragunski, D.C. Effects of a native cassava starch, chitosan, and gelatin-based edible coating over guavas (Psidium guajava L.). ACS Food Sci. Technol. 2021, 1, 1247–1253.
- Eom, H.; Chang, Y.; sil Lee, E.; Choi, H.D.; Han, J. Development of a starch/gum-based edible coating for rice cakes to retard retrogradation during storage. LWT 2018, 97, 516–522.
- Omid Jeivan, A.; Taghizadeh, M.; Yavarmanesh, M. Optimization of edible coating based on modified potato starch and rice bran oil and evaluation of its effect on grape’s shelf life. Innov. Food Technol. 2021, 8, 273–294.
- Ma, Y.; Zhao, Y.; Xie, J.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Optimization, characterization and evaluation of papaya polysaccharide-corn starch film for fresh cut apples. Int. J. Biol. Macromol. 2021, 166, 1057–1071.
- Nogueira, G.F.; de Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of incorporating plant-derived bioactive compounds into films made with agro-based polymers for application as food packaging: A brief review. Polymers 2020, 12, 2518.
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and incorporation of functional ingredients in edible films and coatings. Food Bioprocess Technol. 2020, 14, 209–231.
- Perazzo, K.K.N.C.L.; Conceição, A.C.D.V.; dos Santos, J.C.P.; de Assis, D.J.; Souza, C.O.; Druzian, J.I. Properties and antioxidant action of actives cassava starch films incorporated with green tea and palm oil extracts. PLoS ONE 2014, 9, e105199.
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167.
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Reungsang, A.; Rachtanapun, P. Antioxidant films from cassava starch/gelatin biocomposite fortified with quercetin and TBHQ and their applications in food models. Polymers 2021, 13, 1117.
- Santos, L.S.; Fernandes, C.C.; Santos, L.S.; de Deus, I.P.B.; de Sousa, T.L.; Miranda, M.L.D. Ethanolic extract from Capsicum chinense Jacq. ripe fruits: Phenolic compounds, antioxidant activity and development of biodegradable films. Food Sci. Technol. 2020, 41, 497–504.
- Romani, V.P.; Hernández, C.P.; Martins, V.G. Pink pepper phenolic compounds incorporation in starch/protein blends and its potential to inhibit apple browning. Food Packag. Shelf Life 2018, 15, 151–158.
- Chibane, B.; Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Open archive toulouse archive ouverte plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2018, 99, 1457–1474.
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100.
- Kola, V. Plant Extracts as Additives in Biodegradable Films and Coatings in Active Food Packaging: Effects and Applications. Master’s Thesis, Faculty of Sciences and Technology of University of Algarve, Algarve, Portugal, 2020.
- Rojas-Bravo, M.; Rojas-Zenteno, E.G.; Hernández-Carranza, P.; Ávila-Sosa, R.; Aguilar-Sánchez, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. A potential application of mango (Mangifera indica L. cv. manila) peel powder to increase the total phenolic compounds and antioxidant capacity of edible films and coatings. Food Bioprocess Technol. 2019, 12, 1584–1592.
- Nunes, C.; Maricato, É.; Gonçalves, F.J.; da Silva, J.A.L.; Rocha, S.M.; Coimbra, M.A. Properties of chitosan-genipin films grafted with phenolic compounds from red wine. Trends Carbohydr. Res. 2015, 7, 25–32.
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and applicability of starch-based films: An eco-friendly approach. Foods 2021, 10, 2181.
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent developments in smart food packaging focused on biobased and biodegradable polymers. Front. Sustain. Food Syst. 2021, 5, 125.
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871.
- Santhosh, R.; Nath, D.; Sarkar, P. Novel food packaging materials including plant-based byproducts: A review. Trends Food Sci. Technol. 2021, 118, 471–489.
- Figueirinha, A.; Cruz, M.T.; Francisco, V.; Lopes, M.C.; Batista, M.T. Anti-inflammatory activity of Cymbopogon citratus leaf infusion in lipopolysaccharide-stimulated dendritic cells: Contribution of the polyphenols. J. Med. Food 2010, 13, 681–690.
