The Teplice Program was initiated by the Czech Ministry of Environment. The research program was prepared in collaboration with the United States Environmental Protection Agency (US EPA) and included air pollution monitoring, human exposure, biomarker studies, and health effects studies.
- air pollution
- SO2
- PAHs
- PM2.5
- DNA adducts
- pregnancy outcome
- sperm abnormalities
- neurobehavioral changes
- mortality
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Mining districts in Northern Bohemia, the northern region in the Czech Republic, were in the late 1980s one of the most air polluted regions in Europe. Northern Bohemia is a highly industrialized coal basin. Brown coal containing 1–5% sulphur was used for power plants, industry, and local heating. Geographically, this area is a valley sandwiched between the Ore Mountains approximately 1000 m above sea level to the north and the Middle Bohemia Highlands, approximately 800 m above sea level to the south. The geographic location and prevailing winds from the northwest and southwest give rise to frequent inversions. The average concentration of sulphur dioxide (SO2) in the years 1982–1990 was 103 µg/m3, and that of total suspended particles (TSP) was 102 µg/m3 [1]. Air pollution from these sources caused extensive deforestation of conifers in the Ore Mountains.
Air pollution significantly affected human health. Kotesovec et al. [2] observed that increased daily mortality was related to air pollution for total mortality, cancer, and cardiovascular mortality, and significant shortening of life expectancy by 2 years for males and females. Sram [3] studied the impact of air pollution on pregnancy outcomes, diagnosed as congenital anomalies (CGA) or lower birth weight (LBW) in medical records of maternity hospitals between the years 1982 and 1986. In the district of Usti nad Labem, 7644 pregnancies were diagnosed with 9.8% CGA. In the district of Teplice, 7190 pregnancies were diagnosed with 8.2% CGA. This was approximately 4–5 times higher in this region than in other parts of the Czech Republic, according to official records. Similarly, in the same districts, more children were born with birth weights lower than 2500 g (LBW, 7.5–9.2% vs. cca 4.5% nationwide). Morbidity of children in the mining districts of Northern Bohemia differed significantly from the morbidity nationwide as follows [4]:
-
for children 0–6 years old:
-
respiratory diseases: 2.90 vs. 0.54 nationwide (No. of cases/100),
-
mental illness: 1.06 vs. 0.53 nationwide (No. of cases/100);
-
-
for children 7–15 years old:
-
respiratory diseases: 1.40 vs. 0.45 nationwide (No. of cases/100),
-
mental illness: 4.09 vs.2.00 nationwide (No. of cases/100).
-
The health consequences of environmental pollution became one of the major concerns of the Czech government after political changes in 1989. At the end of 1990, the government put forward an interdisciplinary project later called the Teplice Program in order to analyze the impact of air pollution on human health in the mining districts [5]. The mining district of Teplice in Northern Bohemia was used as the polluted district for this program. The district of Prachatice in Southern Bohemia had some of the cleanest air in the Czech Republic and was used as the control district (Figure 1). The distance between those two districts is 240 km. The Teplice district had 127,500 inhabitants and an area of 469 km2, of which a large part had been devastated by the strip-mining of coal and associated industrialization. The district of Prachatice had 51,500 inhabitants and an area of 1375 km2, of which 52% was woodlands. In 1993, the average PM10 concentrations in Teplice were 76 vs. 38 µg/m3 in Prachatice. Similarly, PM2.5 concentrations were 64 vs. 32 µg/m3, respectively, and benzo[a]pyrene (B[a]P) 3.7 vs. 2.5 ng/m3 [6,7].
2. Teplice Program
2.1. Air Quality Monitoring
Pinto et al. [8] collected aerosol samples from Teplice in February–March and May–July 1992, and from Teplice and Prachatice during three periods: January–March 1993, May–August 1993, and November 1993–March 1994. Ambient aerosol and acidic gas samples were collected by the versatile air pollution sampler (VAPS). The samples were analyzed for three indicators of air pollution: SO2, PM2.5 and B[a]P; not all samples were analyzed for all indicators. These findings are shown in Table 1.
Table 1. Concentrations of indicators of air pollution in Teplice (target population) and Prachatice (control group).
| Indicators of Air Pollution | February–March 1992 | May–July 1992 | January–March 1993 | May–August 1993 | November 1993–March 1994 | |||
|---|---|---|---|---|---|---|---|---|
| Teplice | Teplice | Teplice | Prachatice | Teplice | Prachatice | Teplice | Prachatice | |
| SO2, µg/m3 | 135 ± 20 | 31.1 ± 4.7 | 153 ± 23 | 29.0 ± 4.4 | 4.4 ± 0.7 | |||
| PM2.5, µg/m3 | 68.0 ± 1.9 | 36.5 ± 1.2 | 122 ± 3.1 | 44.0 ± 0.8 | 28.7 ± 1.2 | 17.9 ± 0.4 | 51.1 ± 2.8 | |
| B[a]P, ng/m3 | 8.0 ± 0.4 | 4.7 ± 2.4 | 0.5 ± 0.4 | 0.1 ± 0.05 | 5.5 ± 0.3 | 3.4 ± 0.5 | ||
Pinto et al. [9] later used the ambient monitoring and source characterization data to determine the relative contributions of different source categories to the level of ambient PM2.5 in Teplice and Prachatice (Figure 2).
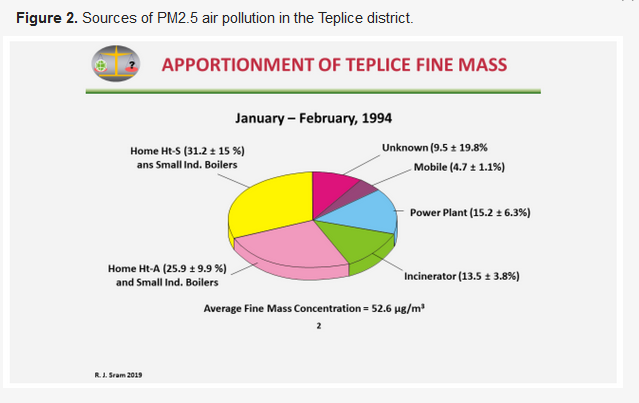
Prior to this analysis, it was believed that the main source of PM2.5 pollution was from power plants. However, in the period with fewer and less severe inversions (January–February 1994), the most significant source was from local heating (31.2% + 25.9% + 13.5% = 70.6%) compared to 15.2% from power plants and 4.7% from mobile sources. What was originally postulated as incinerator activity was in fact waste burned in local heating sources [8].
The results of study of pollution sources were extremely significant: The high SO2 content measured in Teplice was undeniable and was tied to the use of brown coal in local heating. Therefore, at the end of 1994, the Czech government approved 6.2 billion CZK to convert local heating in all the mining districts from brown coal to natural gas. This change substantially decreased air pollution by lowering levels of SO2 as well as PM2.5.
2.2. Genotoxicity and Embryotoxicity of Urban Air Particulate Matter
PM10 were collected daily in Teplice and Prachatice using the HiVol air sampler Anderson equipped with Pallflex filters 20 × 20 cm (TA60A20) during winter (October–March) and summer (April–September) in the years 1993–1994. The organic mass of crude extracts was dissolved in dimethyl sulfoxide (DMSO). The in vitro acellular assay for DNA adducts was analyzed by 32P-postlabeling, while embryotoxicity assay was performed using the Chick Embryotoxicity Screening Test (CHEST) [10,11]. The characteristics of the winter air samples are as follows: Teplice PM10 69.3 µg/m3, B[a]P 7.42 ng/m3; Prachatice PM10 29.6 µg/m3, and B[a]P 5.37 ng/m3. 32P-postlabeling DNA adducts were analyzed via high-performance liquid chromatography (HPLC) to identify some of the major DNA adducts. Using CHEST, embryotoxicity was defined as the sum of dead and malformed embryos. Identified DNA adducts were derived from 9-OH/B[a]P, anti-BPDE, B[b]F, B[j]F, B[k]F, CHRY, B[a]A, and I[c,d]P. The radioactivity of these adduct spots accounted for approximately 50% of total radioactivity detected along the diagonal zone. A good correlation between DNA adduct levels formed in the presence of the S9 metabolic activation system and the dose inducing 50% of exposed embryos malformation/or death was observed (r = 0.773, p < 0.001). In assays from both Teplice and Prachatice, the highest activity was found for fractions containing mainly polycyclic aromatic hydrocarbons (PAHs). These results agreed with those of other studies [12,13] which show that PAHs account for most of the mutagenic activity present in the neutral fraction of urban air.
This was the first report comparing the biological activities of complex mixtures in short-term assays with remarkably different end points, such as DNA adducts formation and embryotoxicity.
The results indicate that PAHs are a major source of genotoxic activities of organic mixtures associated with urban air particles in both districts. The results also confirmed similarities of the major emission sources of organic compounds in both districts which are presumably residential home heating in the winter and motor vehicles in the summer.
2.3. DNA Adducts and Personal Air Monitoring
Binkova et al. [14] analyzed the effect of carcinogenic PAH (c-PAH) exposure on DNA adducts (DNA isolated from WBC (white blood cells) detected by 32P-postlabeling) in a group of 30 healthy women from the city of Teplice. The women worked outdoors as postal workers and gardeners. Personal samplers used for collecting respirable particles PM2.5 (<2.5 µm) were provided by the US EPA [15]. PM2.5 collected on quartz filters was extracted and analyzed by HPLC with fluorometric detection [15]. In the pilot study in November 1992, authors observed a significant difference in DNA adduct levels between smokers and nonsmokers. Sampling in nonsmokers was done on 24 and 26 November, when the concentration of c-PAHs was 14.9 ± 6.9 vs. 7.7 ± 3.3 ng/m3. In addition, the total DNA adduct levels differed significantly on those two days of sampling (5.59 ± 2.96 vs. 2.61 ± 1.40 adducts/108 nucleotides, p < 0.05). Ten women nonsmokers participated in a follow-up study on four sampling days from October 1993 to February 1994 (Table 2). In both studies, there was a significant effect (p < 0.01) of sampling day on DNA adduct levels that was related to personal exposure data. Correlation analysis proved the relationship between c-PAH personal exposure and DNA adduct level (r = 0.621, p < 0.001). Therefore, the authors recommended using simultaneous personal exposure monitoring if WBC are to be used for DNA adduct analysis.
Table 2. DNA adducts in healthy women in Teplice.
| Sampling | |||||
|---|---|---|---|---|---|
| November 1992 | October 1993 | November 1993 | January 1994 | February 1994 | |
| PM2.5 µg/m3 | 53.5 ± 30.5 | 52.8 ± 39.4 | 106 ± 49.9 | 33.3 ± 14.1 | 39.3 ± 46.8 |
| c-PAHs ng/m3 | 12.2 ± 5.6 | 14.5 ± 6.4 | 42.2 ± 19.9 | 21.3 ± 18.5 | 15.1 ± 6.0 |
| B[a]P ng/m3 | 3.0 ± 1.3 | 2.8 ± 12.6 | 7.5 ± 3.6 * | 3.8 ± 4.0 | 2.0 ± 1.1 |
| DNA adducts/108 nucleotides | 5.73 ± 0.90 | 4.64 ± 1.95 | 6.81 ± 1.81 | 4.37 ± 2.05 | 3.96 ± 0.80 |
2.4. Pregnancy Outcome
This study evaluated the impact of air pollution and lifestyle variables on full-term singleton births of European origin in the Teplice district from April 1994 through March 1996 (N = 1943). A total of 190 (9.8%) infants were below the 10th percentile of birth weight for gestational age. Thirty-day averages for PM10 varied from 29 to 86 µg/m3, with a mean of 47.7 ± 12.6 µg/m3. Thirty-day averages for PM2.5 varied from 17 to 70 µg/m3, with a mean 35.7 ± 11.8 µg/m3. Elevated crude odds ratios (ORs) were observed for IUGR (intrauterine growth retardation) in the first month of pregnancy as follows: for PM10, medium 40 to < 50 µg/m3 (OR = 1.62, CI 1.02–2.50, p < 0.02); for PM2.5, high > 50 µg/m3 (OR = 2.64, CI 1.48–4.71, p < 0.001) levels. Results for PM10 and PM2.5 were similar, but only the adjusted OR for the high PM2.5 was statistically significant (OR = 1.68, CI 1.18–2.40, p < 0.05). Increases in IUGR during the first month of gestation were associated with PM10 concentrations over 40 µg/m3 and PM2.5 over 37 µg/m3 in the Teplice district (Table 3). These data suggest that exposure to particulate matter or associated air pollutants early in pregnancy may adversely affect fetal growth [16].
Table 3. Adjusted * odds ratio (AOR) of intrauterine growth retardation (IUGR) for PM10 by month of gestation.
| Month | PM10: 40 to <50 µg/m3 | PM10 > 50 µg/m3 | ||||
|---|---|---|---|---|---|---|
| AOR | CI | p-Value | AOR | CI | p-Value | |
| 1 | 1.62 | (1.07–2.50) | 0.02 | 2.64 | (1.48–4.71) | 0.001 |
| 2 | 1.09 | (0.72–1.63) | 0.69 | 1.01 | (0.60–1.69) | 0.98 |
| 3 | 1.02 | (0.68–1.54) | 0.93 | 0.87 | (0.51–1.47) | 0.59 |
| 4 | 1.27 | (0.85–1.90) | 0.25 | 0.93 | (0.55–1.58) | 0.78 |
| 5 | 0.92 | (0.62–1.36) | 0.66 | 0.82 | (0.48–1.39) | 0.46 |
| 6 | 0.95 | (0.65–1.39) | 0.77 | 0.74 | (0.42–1.30) | 0.29 |
| 7 | 0.83 | (0.57–1.21) | 0.33 | 0.83 | (0.49–1.42) | 0.50 |
| 8 | 1.22 | (0.83–1.79) | 0.31 | 1.16 | (0.66–2.03) | 0.61 |
| 9 | 1.03 | (0.70–1.52) | 0.88 | 1.25 | (0.73–2.12) | 0.42 |
* Adjusted for maternal height, pre-pregnancy weight, completed high school, currently married, month-specific smoking habits, year and season.
One possible explanation for this finding is that co-pollutants such as PAHs may interfere with fetal development as they are usually adsorbed on the surface of fine particles. Binkova et al. [11] observed that genotoxicity of particulate matter in the ambient air is related mainly to PAHs. Another study on the same population from Teplice and Prachatice suggested that DNA–PAH adducts in placentae were positively related to IUGR [17]. Both these studies indicate that PAHs are the major source of genotoxic and embryotoxic activities of organic mixtures associated with air pollution in the Teplice district.
Dejmek et al. [18] further analyzed single births in the Teplice and Prachatice districts in the study from April 1994 to March 1998. Concentrations of PM10 and PM2.5 were continually measured using VAPS. The carcinogenic PAHs (c-PAHs) were identified as chrysene, bez[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenz[a,h]anthracene, and indeno[1,2,3-c,d] pyrene [15]. The pollutant data for each month were divided into low (L), medium (M), and high (H) concentrations, which were the same for PM10 and PM2.5 as described in Dejmek et al. [16]. Concentrations for c-PAHs were L = < 15 ng/m3, M = 15 to <30 ng/m3, H = 30 ng/m3 or higher. In the Teplice study, 3349 pregnancies were evaluated, and IUGR exhibited in 322 (9.6%) newborns. In Prachatice, 1505 pregnancies were evaluated, and IUGR exhibited in 124 (8.2%) newborns. IUGR was notably increased in the first month of gestation in Teplice, ORs for PM10 were M = 2.11 (CI, 1.03–2.02), H = 2.14 (CI, 1.42–3.23). Corresponding values for PM2.5 were M = 1.38 (CI, 0.95–1.92, p < 0.15) and H 1.96 (CI, 1.02–3.11, p < 0.002). In the district of Prachatice, a significant association was observed only for PM10: M = 2.11 (CI, 1.03–4.33). c-PAHs increased IUGR in Teplice in the first gestation month M = 1.59 (CI, 1.06–2.39, p < 0.025), H = 2.15 (CI, 1.27–3.63, p < 0.001) (Figure 3). The risk of an infant born with IUGR increases with the level of fine particles and c-PAHs in the first month of gestation. The association between PM10 and IUGR observed in a previous study by Dejmek et al. [16] may be explained in part by PAHs adsorbed to air particles.
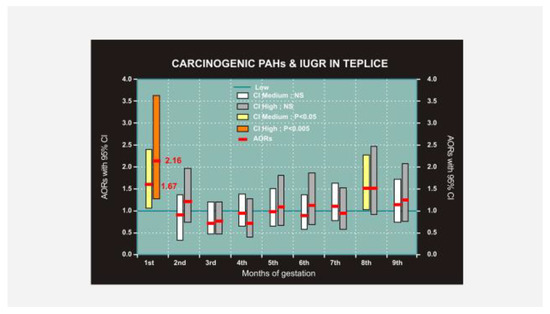
Figure 3. Impact of carcinogenic PAH (c-PAH) exposure to mothers in the Teplice district on IUGR in their newborns.
The effects of PAHs on fetal development and growth may be explained by PAH penetration into the placenta and different fetal tissues [19,20,21,22,23] and by direct interference with placental growth factors [24,25].
According to Barker [26], reduced fetal growth is an important predictor of later adult health risks, such as noninsulin-dependent diabetes, hypertension, and coronary heart disease. Therefore, higher exposure to pollutants during the early stages of intrauterine life may be responsible for diseases in middle age.
Epstein et al. [27] studied the relationship between toxic trace metals and outcomes of first delivery in pregnant women in Teplice and Prachatice. Maternal and cord blood levels of lead, mercury, and cadmium were very low and arsenic was undetectable. No effect of these metals to low birth weight or IUGR was observed.
2.5. Biomarkers and Pregnancy, DNA Adducts
Topinka et al. [17] analyzed DNA adducts in human placenta related to air pollution in nonsmoking mothers from the districts of Teplice and Prachatice. Forty-nine placenta samples were from summer 1994 and forty-nine samples from winter 1994–1995 (each sampling N = 25 from Teplice, N = 24 from Prachatice). They observed 1.40 ± 0.87 and 1.04 ± 0.63 adducts per 108 nucleotides for the Teplice and Prachatice districts, respectively. A significant difference between both districts in placental DNA adduct levels was found only for winter samples (1.49 vs. 0.96 adducts per 108 nucleotides, p < 0.023). Positive glutathione S-transferase M1 (GSTM1) metabolic genotype was detected in 51 mothers, and GSTM1-null genotype was found in 47 subjects. Higher DNA adduct levels were detected in a group with GSTM1-null genotype (p < 0.01). This finding was more significant in the polluted Teplice district (p < 0.05).
In another study with 158 mothers (113 nonsmokers and 45 smokers), DNA adduct levels were significantly higher in the polluted region and in smoking mothers. Using multiple regression models to analyze the effect of c-PAH concentrations and vitamin C levels in nonsmoking mothers, an inverse relationship between vitamin C levels and DNA adduct levels was found (b = −0.513, p < 0.05). Higher DNA adduct levels were observed in nonsmoking mothers delivering children with IUGR (b = −0.741, p = 0.01) [28].
DNA adduct data in placenta related to the effect of c-PAH exposure are complementary with in vitro DNA binding activity and embryotoxicity studies [11]; this proved the genotoxic and embryotoxic potential of the organic extracts from the Teplice and Prachatice districts.
2.6. Semen Quality
Rubes et al. [29] also examined associations between exposure to episodes of air pollution and increased DNA fragmentation in human sperm in young men from Teplice (N = 36), who were sampled up to 7 times between the years 1995 and 1997. No significant associations were found between exposure to air pollution and routine semen testing, in terms of volume, concentration, total count, motile percentage, or percentage of normal morphology. Only sperm chromatin structure (SCSA) changes expressed as DNA fragmentation index (SCSA-%DFI) were significantly associated with exposure to high levels of air pollution as previously indicated by Selevan et al. [30]. In the comparison of air pollution in January 1996 vs. September 1997, PM10 was 52 vs. 25 µg/m3, and PAH was 145 vs. 30 ng/m3, which corresponded to SCSA-% DFI 20.3 (16.0–24.6) vs. 12.2 (9.5–14.8).
Rubes et al. [29] put forward the hypothesis that reactive metabolites of PAHs might reach the testicles and react with sperm DNA to form breaks, which cannot be repaired in epididymal sperm about 10 days before ejaculation. This may be manifested as increased SCSA-%DFI. The study also included measurement of blood lead, cadmium, and mercury, but these blood metals were not associated with air pollution.
These two studies from the Czech Republic [29,30] were the first epidemiological studies reporting associations between air pollution and altered semen quality as sperm chromatin structure.
Rubes et al. [31] later studied the impact of c-PAHs on sperm quality in city policemen in Prague by SCSA. Concentrations of B[a]P in February 2007 were 1.03 ± 0.77 ng/m3 while in May 2007 0.16 ± 0.05 ng/m3. Winter concentrations of B[a]P significantly increased sperm chromatid damage: hDFI was 7.31 ± 3.64% vs. 5.46 ± 3.21% in May. These data were later used for the evaluation of B[a]P health risk by the WHO (World Health Organization) in 2010: personal exposure to B[a]P over 1.0 ng/m3 predict DNA fragmentation in sperm [32].
2.7. Neurobehavioral Studies
The study of the impact of air pollution on a child’s neurodevelopment was started only in the districts of Northern Bohemia. The study analyzed symptoms of minimal brain dysfunction (MBD) in the 5080 children attending the second grade in the districts of Usti nad Labem, Teplice, and Jablonec nad Nisou. Behavioral changes were observed in children in the polluted districts where 4.8% attended special needs schools; for those children who attended the regular schools, 10% were diagnosed with MBD [33].
Therefore, the children living in these districts were at greater risk for learning disorders due to the significant levels of air pollution, especially the high concentration of SO2 in ambient air, compared to other children in the Czech Republic. According to the Czech Statistical Institute [4] in 1988, mental illness was diagnosed in 4.09% of children of the age group 7–15 years old in the mining districts vs. 2% in the Czech Republic. The effect of SO2 exposure during pregnancy in mice by Singh [34] demonstrated changes in behavior for the righting reflex and negative geotaxis as time progressed. Therefore, Sram [3] hypothesized that in utero exposure to environmental chemicals causes functional changes in the nervous system that are expressed as developmental disorders or other behavioral dysfunctions.
Otto et al. [35] assessed neurobehavioral functions using the Neurobehavioral Evaluation System (NES2, computerized assessment battery) [36] in 2nd-, 4th-, and 7th-grade students from Teplice and Prachatice (2nd-grade cohort N = 772, 4th-grade cohort N = 322, 7th-grade cohort N = 470 children) (Table 4).
Table 4. Percent of children referred for assessment of learning disabilities or behavioral problems.
| Cohort | Teplice | Prachatice |
|---|---|---|
| 2nd grade | 26.6 | 12.9 |
| 4th grade | 27.3 | 13.0 |
| 7th grade | 25.6 | 13.1 |
Those results indicate a poorer performance on neurobehavioral tests and high prevalence of learning disabilities in children from the air-polluted mining district of Teplice [35]. Arsenic (As) and mercury (Hg) levels in hair and urine were low and were not associated with any performance measures. SO2 levels were markedly higher in the Teplice mining district. During the critical perinatal period (1982–1983) for the 7th-grade Teplice students, the mean ambient SO2 levels were 145.9 ± 24.6 µg/m3. In January–March 1993, the SO2 level was 153 µg/m3 in Teplice vs. 29 µg/m3 in Prachatice, while PM2.5 was 122 µg/m3 vs. 44 µg/m3. Results of Otto’s work [35] seem to correspond with the original idea about the negative impact of air pollution on neurobehavioral function in children.
Increased concentrations of PAHs in polluted air may affect neuropsychological development in children. Prenatal exposure to PAHs was studied in cohorts of children from New York (USA) [37,38,39,40], Krakow (Poland) [41,42] and Tongliang (China) [43]. All studies observed decreases of cognitive function, intelligence quotient, and decrease of white matter volume in the left hemisphere. Therefore, it may be hypothesized that changes in neurobehavioral function in the population in Northern Bohemia may be a consequence of the high concentration of PAHs present in the mining districts in the decades prior to the Teplice Program.
2.8. Mortality
Life expectancy in the district of Teplice in 1988 was 64.9 years for males and 73.9 years for females compared to 68.2 years and 75.4 years, respectively, in the Czech Republic [2]. Now, life expectancy in the Czech Republic is 76 years for males and 82 years for females, but in the mining districts, it is 2 years shorter for males as well as females [44]. The extension of life expectancy after political changes in 1989 was related to improvements in the health care system and the shift in lifestyles and disease awareness [45]. Modern health policies included screening programs for certain malignant neoplasms, tobacco/smoking bans in public places and atialcohol measures [46]. Declines in cardiovascular mortality were associated with improvements in prevention and/or treatment of ischemic heart disease [47].
Kotesovec and Skorkovsky [48] analyzed mortality in the mining districts (Chomutov, Most, Teplice, Usti nad Labem, and Decin) which had a total of 620,000 inhabitants in the period of high (1982–1994) and decreased pollution (1995–2004). The concentration of SO2 in those two periods was 93.09 vs. 22.73 µg/m3, and PM10 was 97.00 vs. 44.14 µg/m3. The total standardized mortality in the period of high pollution was 14.80/1000 for males and 13.60/1000 for females; cardiovascular mortality 7.45/1000 for males and 8.17/1000 for females; and respiratory mortality 0.85/1000 for males and 0.52/1000 for females. Total standardized mortality in the period of decreased pollution was 13.10/1000 for males and 12.25/1000 for females; cardiovascular mortality 6.33/1000 for males and 7.18/1000 for females; and respiratory mortality 0.56/1000 for males and 0.42/1000 for females. Comparing those two periods, total mortality decreased by 5.1% (4.2, 6.1) for males and by 2.7% (1.9, 3.6) for females; cardiovascular mortality decreased by 6.9% (5.6, 8.3) for males and by 3.7% (2.6, 4.9) for females; and respiratory mortality decreased by 17.8% (13.8, 21.9) for males and by 5.5% (1.2, 9.9) for females. The most significant decrease was observed with cardiovascular mortality in subjects under 60 years old: for males by 11.3% (8.5, 14.2) and for females by 12.2% (7.6, 17.1).
The substantial decrease of air pollution in the mining districts between the years 1995 and 2004 significantly decreased total, cardiovascular, and respiratory morbidity in both males and females. Kotesovec and Skorkovsky [48] calculated that within that period, 195 fewer males and 92 fewer females were dying each year, totaling 1950 males and 920 females living longer. These results prove the significant implication that decrease in air pollution has on the health of the people in the mining districts.
3. Conclusions
In evaluating the Teplice Program after more than 20 years, it may be said that it was truly a unique international project. In collaborating closely with the US EPA, Czech scientists were trained in many new methodologies used in health studies. The results were unexpected in some cases and in other cases affirmed what various professionals had already suspected. The totality of the results is no less profound and is summarized below.
(1) In analyzing the sources of air pollution, approximately 70% of PM2.5 fine particles were attributed to local heating sources that used brown coal containing a high content of SO2. This result prompted the Czech government in 1994 to support the change of local heating in the mining districts from using coal to natural gas. This substantially decreased the concentration of SO2 and PM2.5 in the region;
(2) In vitro studies, evaluated in terms of the level of DNA adducts, proved that PM10 extracts contained many c-PAHs and that those c-PAHs contributed to 50% of the genotoxicity of PM10 in the region;
(3) The use of personal monitors and determining the level of DNA adducts in WBC of exposed subjects showed for the first time the relationship between c-PAH exposure and the impact on DNA adducts;
(4) In the pregnancy outcome study, analyzing several thousand pregnancies over 4 years showed that the first month of gestation is the most sensitive to IUGR induction. The prevalence of IUGR was shown to be related to the concentration of c-PAHs > 15 ng/m3 (B[a]P > 2.8 ng/m3) adsorbed on PM2.5. This reduced fetal growth may substantially affect later adult health;
(5) For the first time, the impact of air pollution on DNA fragmentation in sperm was demonstrated in the semen study;
(6) In the neurobehavioral studies, poorer performance on neurobehavioral tests and high prevalence of learning disabilities in children from the polluted district was observed. The studies postulated a significant impact of air pollution that affects neurobehavioral function in children;
(7) The mortality studies observed a significant decrease of life expectancy, approximately 2 years for males and females. Decreased air pollution later significantly decreased cardiovascular and respiratory mortality in mining districts;
(8) It may be suspected that the health of the population in the mining districts of Northern Bohemia is significantly affected by decades of air pollution. High concentrations of c-PAH induced genetic damage as well as affected birth weight, which later exhibits as functional changes that increase morbidity. This damage seems to be long-lasting and can extend through an entire lifetime.
These facts should be used to prepare a new “Teplice Program” to learn the present quality of life in the mining districts and use new knowledge to prepare a preventive program to improve the population’s health in this region.
- Skorkovsky, J.; Kotesovec, F. Comparison of mortality in the industrial region of Northern Bohemia in the period of higher and lower level of air pollution (In Czech). Air Pollution Protection 2005, 18, 5-6: 32-37.
- Kotesovec, F.; Skorkovsky, J.; Brynda, J.; Peters, A.; Heinrich, J. Daily mortality and air pollution in Northern Bohemia: different affects for men and women. Centr Eur J Publ Health 2000, 8, 120-127.
- Sram, R.J. New ethical problems related to environmental pollution. In: Ethical Issues of Molecular Genetics in Psychiatry. Sram, R. J., Bulyzhenkov, V, Prilipko, L, Christen, Y, Eds.: Springer Verlag: Berlin-Heidelberg, Germany, 1991, pp. 94-105.
- Czech Statistical Office 2018 (http://www.czso.cz, April 17, 2019
- Sram, R. J.; Benes, I.; Binkova, B.; Dejmek, J.; Horstman, D. et al. Teplice Program - The impact of air pollution on human health. Environ Health Perspect 1996, 104 (Suppl. 4), 699-714.
- Benes, I.; Skorkovsky, J.; Novak, J.; Sram, R. J. The development of air pollution in Teplice, Prachatice and Prague in the last thirteen years (In Czech). Air Pollution Protection 2007, 20, No. 5-6: 9-10.
- CHMI (Czech Hydrometeorology Institute) 2018. http://portal.chmi.cz/files/portal/docs/uoco/isko/tab_roc/2018_enh/pollution_my/CZTOS_BaP_CZ.html, June 18, 2019.
- Pinto, J.P.; Stevens, R. K.; Willis, R. D.; Kellog, R.; Mammane, Y. et al. Czech air quality monitoring and receptor modelling study. Environ Sci Technol 1998, 32, 843/854.
- Pinto, J.P.; Stevens, R. K.; Willis, R. D.; Mamane, Y.; Ramadan, Z. et al. Source-receptor relations in Teplice and Prachatice. In: Teplice Program - Impact of Air Pollution on Human Health. Sram, R. J. Ed.: Academia: Prague, Czech Republic, 2001, pp. 71-80.
- Binkova, B.; Lenicek, J.; Benes, I.; Vidova, P.; Gajdos, O. et al. Genotoxicity of coke-oven and urban air particulate matter in in vitro acellular assays coupled with 32P-postlabeling and HPLC analysis of DNA adducts. Mutat Res 1998, 414, 77-94.
- Binkova, B.; Vesely, D.; Vesela, D.; Jelinek, R.; Sram, R. J. Genotoxicity and embryotoxicity of urban air particulate matter collected during winter and summer period in two different districts of the Czech Republic. Mutat Res 1999, 440, 45-58.
- De Marini, D.M.; Shelton, M. L.; Bell, D. A. Mutation spectra in Salmonella of complex mixtures: comparison of urban air to benzo[a]pyrene. Environ Mol Mutagen 1994, 24, 262-275.
- De Marini, D.M. Mutation spectra of complex mixtures. Mutat Res 1998, 411, 77-94.
- Binkova, B.; Lewtas, J.; Miskova, I.; Lenicek, J.; Sram, R. DNA adducts and personal air monitoring of carcinogenic polycyclic aromatic hydrocarbons in an environmentally exposed population. Carcinogenesis 1995, 16, 1037-1046.
- Watts, R.; Lewtas, J.; Stevens, R.; Hartlage, T.; Pinto, J. et al. Czech-US EPA health study: assessment of personal and ambient air exposures to PAH and organic mutagens in the Teplice district of Northern Bohemia. Internat J Environ Anal Chem 1994, 56, 271-287.
- Dejmek, J.; Selevan, S. G.; Benes, I.; Solansky, I.; Sram, R. J. Fetal growth and maternal exposure to particulate matter during pregnancy. Environ Health Perspect 1999, 107, 475-480.
- Topinka J.; Binkova, B.; Mrackova, G.; Stavkova, Z.; Benes, I. et al. DNA adducts in human placenta as related to air pollution and to GSTM1 genotype. Mutat Res 1997, 390, 59-68.
- Dejmek, J.; Solansky, I.; Benes, I.; Lenicek, J.; Sram, R. J. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect 2000, 108, 1159-1164.
- Huel, G.; Girard, F.; Frery, N.; Godin, J.; Blot, P. et al. Placental aryl hydrocarbon hydroxylase activity and placental calcification. Toxicology 1992, 71, 257-256.
- Huel, G.; Godin, J.; Frery, N.; Girard, F.; Moreau, T. et al. Aryl hydrocarbon hydroxylase activity in human placenta and threatened preterm delivery. J Expo Anal Environ Epidemiol 1993, 3 (suppl 1), 187-189.
- Hatch, M. C.; Warburton, D.; Santella, R. M. Polycyclic aromatic hydrocarbon-DNA adducts in spontaneously aborted fetal tissue. Carcinogenesis 1990, 11, 1673-1775.
- Madhavan, N.D.; Najdu, K. Polycyclic aromatic hydrocarbons in placenta, maternal blood, umbilical cord blood and milk of Indian women. Hum Exp Toxicol 1995, 14, 503-506.
- Arnould, J. P.; Verhoest, P.; Bach, V.; Libert, J. P.; Belegaud, J. Detection of benzo[a]pyrene -DNA adducts in human placenta and umbilical cord blood. Hum Exp Toxicol 1997, 16, 716-721.
- Guyda, H. J. Metabolic effects of growth factors and polycyclic aromatic hydrocarbons on cultured human placental cells of early and late gestation. J Clin Endocrinol Metab 1991, 72, 718-723.
- Zhang, I.; Connor, E. E.; Chegini, N.; Shiverick, K. T. Modulation by benzo[a]pyrene of epidermal growth factor receptors, cell proliferation, and secretion of human chorionic gonadotropin in human placental lines. Biochem Pharmacol 1995, 50, 1171-1180.
- Barker, D. J. Adult consequences of fetal growth restriction. Clin Obstet Gynecol 2006, 49, 270-283.
- Epstein, H.; Dejmek, J.; Subrt, P.; Vitnerova, N.; Sram, R. J. Trace metals and pregnancy outcome in the Czech Republic. Environ Epidemiol Toxicol 2000, 2, 13-19.
- Sram, R. J.; Binkova, B.; Rossner, P.; Rubes, J.; Topinka, J. et al. Adverse reproductive outcomes from exposure to environmental mutagens. Mutat Res 1999, 428, 203-215.
- Rubes, j.; Selevan, S. G.; Evenson, D. P., Zudova, D.; Vozdova, M. et al. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum Reprod 2005, 20, 2776-2783.
- Selevan, S.G.; Borkovec, L.; Slou, V. L., Zudova, Z.; Rubes, J. et al. Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environ Health Perspect 2000,108, 887-894.
- Rubes, J.; Rybar, J.; Prinosilova, P.; Veznik, Z.; Chvatalova, I. et al. Genetic polymorphisms influence the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutation Res 2010, 683, 9-15.
- Choi, H.; Harrison, R.; Komulainen, H.; Delgado Saborit, J. M. Polycyclic aromatic hydrocarbons. In: WHO Guidelines for Indoor Air Quality. Selected Pollutants. World Health Organization Europe, WHO Regional Office for Europe, Copenhagen, Denmark, 2010, pp. 289-345.
- Gebhart, J. A.; Dytrych, Z.; Tyl, J.; Sram, R. J. On the incidence of minimal brain dysfunction syndrome in children (In Czech). Cs Psychiat 1990, 86, 1-6.
- Singh, J. Neonatal development altered by maternal sulphur dioxide exposure. Neurotoxicology 1989, 10, 523-527.
- Otto, D.; Skalik, I.; Kenneth Hudnell, H.; House, D.; Sram, R. J. Neurobehavioral effects of exposure to environmental pollutants in Czech children. In: Teplice Program - Impact of Air Pollution on Human Health. Sram, R. J. Ed.: Academia: Prague, Czech Republic, 2001, 217-242.
- Baker, E.; Letz, R.; Fidler, A.; Shalat, S.; Plantamura, D. et al. A computer-based neurobehavioral evaluation system for occupational and environmental epidemiology: methodology and validation studies. Neurotoxicol Teratol 1985, 7, 369-377.
- Perera, F. P.; Rauh, V.; Whyatt, R. M.; Tsai, W.Y.; Tang, D. et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner/city children. Environ Health Perspect 2006, 114, 1287-1292.
- Perera, F. P.; Li, Z.; Whyatt, R.; Wang, S.; Camann, D. et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics 2009, 124, e195-e202.
- Perera, F. P.; Tang, D.; Wang, S.; Vishnevetsky, J.; Zhang, B. et al. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6-7 years. Environ Health Perspect 2012, 120, 921-926.
- Peterson, B.S.; Rauh, V. A.; Bansai, R.; Hao, X.; Toth, Z. et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 2015, 72, 531-540.
- Edwards, S. C.; Jedrychowski, W.; Butscher, M.; Camann, D.; Kieltyka, A. et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect 2010, 118, 1326-1331.
- Jedrychowski, W. A.; Perera, F. P.; Camann, D.; Spengler, J.; Butscher, M. et al. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ Sci Pollut Res 2015, 22, 3631-3639.
- Perera, F.; Li, T. Y.; Zhou, Z. J.; Yuan, T.; Chen, Y. H. et al. Benefits of reducing prenatal exposure to coal-burning pollutants to children’s neurodevelopment in China. Environ Health Perspect 2008, 116, 1396-1400.
- WHO Statistical Information System, Czech Republic. [assessed August 26, 2020].
http://www.who.int/countries/cze/en
- Cifkova, R.; Skodova, Z.; Bruthans, J.; Adamkova, V.; Jozifova, M. et al. Longitudinal trends in major cardiovascular risk factors in the Czech population between1985 and 2007/8. Czech MONICA and Czech post MONICA. Atherosclerosis 2010, 211, 676/681.
- Fihel, A.; Pechholdova, M. Between ‘pioneers’ of the cardiovascular revolution and its ‘late followers’: mortality changes in the Czech Republic and Poland since 1968. Eur J Populatin 2017, 33, 651/678.
- Mackenbach, J. P.; Karanikolos, M.; Beral, J. M.; Mckee, M. Why did life expectancy in Central and Eastern Europe suddenly improve in the 1990s? An analysis by cause of death. Scand J Public Health 2015, 43(8), 796/801.
- Kotesovec, F.; Skorkovsky, J. Comparison of mortality in North-Western Bohemia in two periods of high and low air pollution (In Czech). Air Pollution Protection 2007, 20, 5-6: 19-23.
- Dejmek, J.; Solansky, I.; Podrazilova, K.; Sram, R. J. The exposure of non-smoking and smoking mothers to environmental tobacco smoke during different gestational phases and fetal growth. Environ Health Perspect 2002, 110, 601-606.
- Sram, R. J.; Binkova, B.; Dostal, M.; Merkerova-Dostalova, M.; Libalova, H. et al. Health impact of air pollution to children. Int J Hyg Environ Health 2013, 216, 533-540.
- Rossnerova, A.; Tulupova, E.; Tabashidze, N.; Schmuczerova, J.; Dostal, M. et al. Factors affecting 27K DNA methylation pattern in asthmatic and healthy children from locations with various environments. Mutat Res 2013, 741-742, 18-26.
- Sram, R. J.; Rossner, P.; Rossnerova, A.; Milcova, A.; Ambroz, A. et al. Impact of air pollution to genome of newborns. In: ISEE Conference Abstracts, Vol. 2016, Issue 1, 17 Aug 2016, Abstract Number:O-076 (https://ehp.niehs.nih.gov/doi/10.1289/isee.2016.4303).
- Ambroz, A.; Vlkova, V.; Rossner, P., Jr.; Rossnerova, A.; Svecova, V. et al. Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns. Int J Hyg Environ Health 2016, 219, 545-556.
- Honkova, K.; Rossnerova, A.; Pavlikova, J.; Svecova, V.; Klema, J. et al. Gene expression profiling in healthy newborns from diverse localities of the Czech Republic. Environ Mol Mutagen 2018, 59, 401-415.
- Urbancova, K.; Lankova, D.; Rossner, P.; Rossnerova, A.; Svecova, V. et al. Evaluation of 11 polycyclic aromatic hydrocarbon metabolites in urine of Czech moths and newborns. Sci Total Environ 2017, 577, 212-219.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph17186454