Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
A brain tumor, a tumor that develops within the skull, is an abnormal mass of tissue in which cells grow and multiply out of control. Although more than 150 types of brain tumors have been reported, they are macroscopically divided into primary and metastatic groups. Tumors that arise directly from the brain tissue or surrounding the brain are classified as primary brain tumors.
- glioblastoma multiform (GBM)
- photosensitizer (PS)
- reactive oxygen species (ROS)
- surgical resection
- radiotherapy
- chemotherapy
- tumor microenvironment
- blood–brain barrier (BBB)
1. Strategies to Improve Permeability of Nanocarrier through the Blood-Brain Barrier
One of the major limitations of treating brain tumors is the difficulty of delivering drugs to the brain. The brain is surrounded by the blood–brain barrier (BBB), a selective barrier formed by endothelial cells in the cerebral microvessels, which regulates nutrient and ion transport and protects the brain from neurotoxic molecules to maintain brain homeostasis [1]. Unfortunately, most drugs cannot cross the BBB via physiological pathways due to the extreme selectivity of the barrier, which constitutes the greatest obstacle to systemic treatment for most central nervous system (CNS) diseases. In the recent decade, many strategies have been studied, such as topical delivery, implantation of a sustained drug-release scaffold [2], nasal administration [3], ultrasound to temporarily open the BBB [4], and nanoparticle functionalization to enhance BBB penetration [5]. However, local drug delivery methods are considered highly invasive because they require procedural surgery. In addition, the intranasal route has a disadvantage in that the delivered dose varies greatly depending on the condition of the nasal mucosa. Therefore, despite the difficulties across the BBB, the most popular and well-studied delivery route remains the systemic route through the functionalization of nanoparticles.
Nanocarriers can traverse the BBB using a variety of physiological pathways, including receptor-mediated transcytosis (RMT) or adsorption-mediated transcytosis (AMT). To achieve this goal, many nanocarrier systems, such as inorganic, polymeric, or lipid-based nanoparticles, have been developed and shown to cross the BBB due to their tailored surface properties. Numerous studies have demonstrated that physically coating nanoparticles with surfactants and chemical functionalization with specific ligands is a successful strategy to enhance BBB traversing via the physiological pathways mentioned above [6][7]. The size and charge of nanoparticles are also aspects that can affect brain penetration, but if the surface functionalization is done properly, there is no significant difference in a wide size range (from 5 to 400 nm) [8]. Smaller nanoparticles can cross the BBB more easily and diffuse better through the brain, but larger nanoparticles can also cross the BBB in slightly smaller amounts when properly functionalized. On the other hand, larger particles can load a greater amount of drug but reach the brain at a lower concentration, and smaller nanoparticles cannot contain a large amount of drug but reach the brain at a higher concentration. Therefore, the key to increasing the amount of drug delivered to the brain is finding the optimal particle size and designing a nanoparticle system that fits the purpose.
2. Nanotechnology for Enhanced Photodynamic Therapy
Nanotechnology generally involves development of materials with dimensions between 1 and 100 nm, a scale at which the properties of materials differ significantly from those of bulk materials and can be tailored to the desired application [9]. These new chemical and physical properties are usually derived from rapidly increasing surface-to-volume ratios and are associated with plasmonic and quantum effects. With rapidly advancing nanotechnology, nanomaterials are excellent therapeutic and diagnostic tools, and thousands of new compounds and nanostructures are developed each year for diverse applications [10]. This approach using nanotechnology could help overcome several obstacles that have prevented photodynamic therapy from attaining widespread clinical success. The nanostructures studied so far have been applied as a drug delivery platform for PDT and as a strategy to improve the efficiency of photosensitizers that generate ROS upon irradiation. Nanoparticles can be made up of a variety of components, organic and inorganic; can have a variety of shapes and sizes within the nanoscale scale and can act as photosensitizers or as energy converters [11]. Moreover, nanocarriers prevent aggregation caused by the low solubility of photosensitizers in aqueous media such as blood and bypass healthy tissues to increase tumor accumulation. In this section, we discuss cases where nanocarriers provide sufficient therapeutic efficacy and address issues such as undesirable biodistribution or rapid drug clearance from tumor areas.
2.1. Recent Advances in Preclinical Application of Nanocarriers for PDT
Although modern PDT has significantly improved the quality of life and increased overall survival of cancer patients, it is important to further improve the therapeutic effects of nanocarriers to minimize notable side effects such as hydrophobic PS and off-target side effects. In this regard, researchers have studied numerous nanocarriers such as polymers, liposomes, micelles, inorganic oxide, and novel metal nanoparticles to increase the therapeutic efficacy of photosensitizers. First and foremost, it is important to utilize nanocarriers to efficiently deliver photosensitizers and generated singlet oxygen molecules to the target site in an optimal therapeutic range. The pharmacokinetic or pharmacodynamic characteristics of the nanocarriers should be confirmed for clinical use. Therefore, these are being studied for diagnostic as well as photodynamic/chemotherapeutic application using multifunctional nanoparticles.
2.2. Self-Assembled NP via Transformation into Amphiphilic PS-Derivatives
Second-generation PSs have a variety of functional groups including carboxyl, hydroxyl and amine groups in addition to their basic porphyrin structure, allowing hydrophobic modifications via chemical or physical approaches to form amphiphilic PS derivatives. The PS derivatives synthesized to be amphiphilic can form NPs with various nanostructures such as micelles [12][13][14][15][16], PS-drug conjugates [17][18][19][20][21], polymersomes [22], and nanogels [23][24] through self-assembly. According to the nanostructure, nanoparticles can be generally categorized into three types: 1) NPs with mixed hydrophilic and hydrophobic domains, 2) NPs with a core-shell structure, and 3) NPs with a double-layered capsule structure (Figure 1). In this respect, natural polysaccharides such as hyaluronic acid (HA) [24][25][26][27], chitosan [24][28][29][30], chitin, heparin [13][20][21], and fucoidan [23] have been utilized as potential photosensitizer carriers due to their biocompatibility and biodegradability. In addition, not only polymers but also hydrophobic small molecule anticancer drugs (such as doxorubicin [14], docetaxel [15][29][31][32], paclitaxel [33][34][25][35][36], camptothecin, and quercetin [37][38]) can be grafted onto PS.
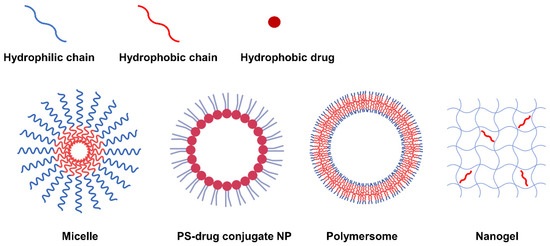
Figure 1. Representative types of NPs classified according to nanostructure.
2.2.1. Self-Assembly Methods for Amphiphilic PS Derivatives
Until recently, various approaches have been tried and developed to promote the self-assembly behavior of amphiphilic PS derivatives. In general, the dispersion method is a suitable method for an amphiphilic material having high water solubility to prepare nanoparticles with a core-shell structure [39]. The process can include mechanical agitation, mild heating or sonication. As an alternative to amphiphilic PS derivatives with low solubility in aqueous media, dialysis has been the most reported method [40][41]. On this basis, the PS derivative can be dissolved in an organic solvent such as dimethyl sulfoxide, dimethyl formamide, or methanol mixed with water and dialyzed against an aqueous solution to remove the solvent. Alternatively, the emulsion method, in which the drug is encapsulated in the oil phase of an oil-in-water emulsion, has received much attention because the drug-loading system has a high loading efficiency [42]. In general, controlled drug release was achieved with the development of dual emulsion technology via water-in-oil in water emulsions [43]. For instance, the structural design of nanocarriers using polyethylene glycolated poly(lactide-co-glycolide) (PEG-PLGA) were obtained as the most suitable approach in nanoemulsions, such as a water-in-oil-in-water evaporation process. In the hydrophilic part of the nanocarrier, cisplatin, a cell proliferation inhibitor, was placed and encapsulated by placing the hydrophobic porphyrin photosensitive dye verteporfin in the oil phase. As a result, PLGA nanocarriers were enabled to efficiently deliver hybrid cargo to cancer cells and PDT-supported enhanced apoptosis.
2.2.2. Carboxyl Group Modification of PS-Derivatives
The carboxyl modification of PS for linking to hyaluronic acid, chitosan, and heparin is mainly achieved through esterification or amidation. Esterification is used to join hydroxyl groups from PS to carboxyl groups in modifiers mediated by coupling agents or catalysts such as dicyclohexyl carbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) (Figure 2a). Most carbodiimides are nonhydrophilic, limiting their application in hydrophilic systems of PS, with the exception of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), which is extensively used in PS preparation. Another approach for modifying PS is to form an amide bond between the amine group of the hydrophobic material and the carboxyl group of the PS, where EDC and N-hydroxysuccinimide (NHS) are commonly used as condensing agents and catalysts, respectively (Figure 3a).
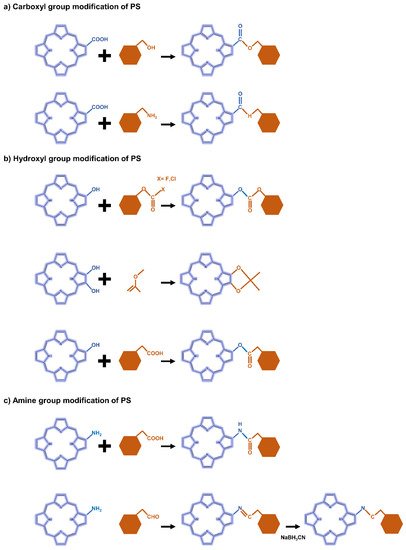
Figure 2. Representative reactions to modify the functional groups of PS-derivative based on (a) carboxyl, (b) hydroxyl, and (c) amine.
2.2.3. Hydroxyl Group Modification of PS-Derivatives
In the case of hydroxyl groups, there are several representative reactions such as etherification and esterification that occur in the presence of alkylating or acylating agents (Figure 2b). As an example, light-controllable host–guest supramolecular amphiphilic complexes between azobenzene mediation porphyrin (TPP-Azo) were synthesized by esterification between TPPC6-COOH and AzoC6-OH [44]. Moreover, dextran alkyl carbonates were synthesized using various types of acylating agents such as butyl chloroformate, butyl fluoroformate, and ethyl chloroformate [45]. A cholesteryl hemisuccinate grafted hyaluronan synthesized through esterification of the carboxyl and hydroxyl groups of hemisuccinate-modified cholesteryl in the presence of DCC/DMAP has also been reported [46].
2.2.4. Amine Group Modification of PS-Derivatives
Heparin [47] and carboxymethyl chitosan [48] of the adipic acid dihydrazide(ADH)-modified polysaccharide type were rendered hydrophobic directly by conjugation of the amino group with the carboxylate moiety of PS via an amino bond in the presence of a catalyst such as EDC/NHS. Another typical strategy is a condensation reaction where the amine group of PS reacts with a carbonyl compound to form an imine intermediate and then is reduced under NaBH3CN (Figure 2c). For these synthetic methods, the most important consideration is the determination of suitable solvents for both hydrophilic PS and hydrophobic molecules.
Chitosan has functional groups such as hydroxyl and amine; therefore, it can be easily modified and crosslinked with other polymers. Of particular benefit in terms of drug delivery, the amino groups of chitosan can be protonated in acidic environments, leading to pH-responsive behavior favored by acidic intracellular organelles such as endosomes and lysosomes. Therefore, chitosan-based nanoparticles have aroused great interest in the field of bio-nanomedicine, especially drug delivery. A dual reactive nanosystem comprised of indocyanine green (ICG) loaded mesoporous silica nanoparticles covered with ZnO quantum dots and coated with erlotinib-modified chitosan for synergistic photodynamic/molecular targeted therapy has been reported. The nanosystem showed a fairly distinct distribution in various nonsmall cell lung cancer models, with favorable anticancer results [49]. Moreover, biodegradable polymer nanoparticles based on chitosan that conjugate various amounts of the photosensitizer tetraphenylchlorin have been developed. These nanoparticles showed high drug loading efficiency and strong retention due to hydrophobic interactions such as π-π stacking between the aromatic photosensitizer group of the polymer and the drug. Nanoparticles have an excellent photodynamic therapeutic effect through photo-induced photochemical activation through high-dose drug delivery, and thus have a strong therapeutic effect on breast cancer cells [50].
2.2.5. Hyaluronic Acid-Modified NPs for PDT
HA is rich in functional groups including carboxyl, hydroxyl and N-acetyl groups, is ready to be transformed into a hydrophobic material, and has a negative charge that can provide a binding platform for hydrophobic macromolecules with a positive charge. Notably, its bioactivity binding to receptors upregulated in cancer cells, such as the cluster determinant 44 (CD44) receptor, the HA-mediated motility receptor (RHAMM), and the lymphatic endothelial (LYVE)-1, allows it to be used for targeted therapeutics. Therefore, HA can act as both a carrier and a target receptor, and HA-based NPs have been extensively studied in the field of drug delivery. HA-related nanosystems (AuNCs-HA) for decorating gold nanocages were developed and exhibited significant photocatalytic properties for PDT, large surface areas, and photothermal therapy (PTT) or PDT properties under near-infrared (NIR) stimulation. In vivo assays showed complete inhibition through the combination of PDT and PTT in AuNCs-HA-treated tumor cells than when each therapy was treated individually [51]. In another study, 5-ALA, Cy7.5 and anti-HER2 antibodies were conjugated to HA and mounted on a gold nanorod (GNR) surface to yield multifunctional GNR-HAALA/Cy7.5-HER2 nanoplatform. As a result, the tumor targeting by HER2 was improved, and side effects were minimized, and the combination of PDT and PTT mediated by 5-ALA and Cy7.5 effectively caused tumor regression [52].
2.3. Application of Inorganic Nanomaterials in PDT
2.3.1. Silica Nanoparticles
Although silica lacks PDT activity on its own, silica nanoparticles can be used to encapsulate PS in PDT due to the chemically inert, nontoxic, and optically transparent nature of silica [53]. In addition, it is commonly used for drug delivery in research because it is possible to functionalize chemicals to the silica through the hydroxyl groups on the silica surface (Figure 3) [54][55]. Mesoporous silica nanoparticles (MSNs) have been extensively utilized to deliver PSs, typically due to their interesting features such as large surface area and pore volume as well as high chemical stability [56][57][58]. One group has developed mesoporous silica-based nanoparticles to exploit continuous oxygen evolution to enhance the effectiveness of PDT treatment in hypoxic cancer environments [57]. To assemble Fe3O4 nanocrystals on silica nanoparticles doped with mesoporous dye, the surface was treated with 3-aminopropyltriethoxysilane and functionalized with amine groups. The oleic acid-stabilized Fe3O4 nanocrystals synthesized in an organic medium were reacted with the amine group of 2-bromo-2-methylpropionic acid, and the resulting Fe3O4 nanocrystals were assembled on the MSN surface by direct nucleophilic substitution between terminal bromine groups. The synthesized biocompatible manganese ferrite nanoparticle-immobilized mesoporous silica nanoparticles alleviated the hypoxic state of tumors with only a small number of nanoparticles and improved the treatment outcome of PDT in vivo.
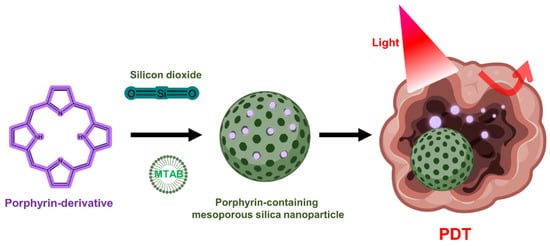
Figure 3. Porphyrin-containing mesoporous silica nanoparticles for PDT.
NIR light-reactive multifunctional nanoparticles are ferrocene-modified with ICG rods and β-cyclodextrin (β-CD) capping for cooperative chemo-dynamic/photothermal/photodynamic (CDT/PTT/PDT) NPs made of mesoporous silica [56]. As a mechanism of chemo-dynamic therapy, ferrocene released from multifunctional nanoparticles was able to efficiently kill cancer cells by converting intracellular H2O2 into toxic OH through a ferrocene-mediated Fenton reaction. Moreover, 1O2 generated by ICG from near-infrared irradiation can kill cancer cells in cooperation with PDT. The results of in vitro experiments show that the CDT/PTT/PDT collaboration significantly amplified the inhibition rate of HeLa cells.
It was reported in one study that silica nanoparticles modified with folic acid (FA) could enhance the site-specific delivery of PS chlorin e6 (Ce6) [59]. By improving the efficiency of targeted drug delivery by FA, efficient generation of singlet oxygen at 670 nm irradiation was obtained, which improved the killing efficacy of NPs on MDA-MB-231 cells compared to free Ce6. Furthermore, a perfluoro hexane (PFH)-encapsulated MSN-based multifunctional nanoplatform using the PS ICG loaded into a polydopamine (PDA) layer and PEG-FA decoration was presented [60]. When excited with 808 nm light irradiation, it mediates the vaporization of PFH, creating bubbles for tumor ultrasound imaging and simultaneously inducing burst drug release. The PTT effect was exerted on the PDA layer, and the loaded ICG was able to generate ROS, a PDT mechanism, while providing NIR fluorescence emission.
2.3.2. Gold Nanoparticles
Gold nanoparticles have been studied for many years for effective PDT induction as well as drug carriers due to promising properties such as high surface area, facile surface modification through gold thiol chemistry, and biocompatibility. Furthermore, gold nanoparticles are being extensively studied for diagnostic applications because of their ability to tune optical scattering and absorption via physical features such as surface plasmon resonance effects [61]. Gold nanoparticles can be applied to PDT without the use of an organic PS. The first use of gold nanorods (AuNRs) alone was reported in 2014 [62]. Upon excitation with relatively long-wavelength NIR light (915 nm), gold nanorods were able to generate a singlet oxygen (1O2) and destroy B16F0 melanoma tumors in mice. Excitation of gold nanorods at a wavelength of 780 nm (λ2), at which the PTT effect can be expected after generation of 1O2, increases the temperature around the tumor tissue, as confirmed by formation of heat shock protein (HSP 70) in which photon energy is converted into heat. By changing the activation wavelength band, the dominant phototherapeutic effect can be switched between PDT and PTT and a synergistic effect can be obtained. It was also possible to trace the distribution of gold nanorods in vivo through self-emitting single-photon-induced fluorescence.
The same group tested the effect of PDT by comparing different types of gold nanoshells, including nanorod-in-shell, nanocage and nanoparticle-in-shell, and demonstrated that it could completely eliminate solid tumors in mice [63]. They can modulate and switch the dominant roles of PDT and PTT by altering the activation wavelength that can excite the gold nanocage. As the most optimal conditions suggested by them, the nanocages mostly showed PDT effect when excited by 980 nm light, whereas 808 nm irradiation induced effective PTT. In vivo studies at 940 nm excitation, a wavelength band between 980 nm and 808 nm, demonstrate that gold nanoshells could induce dual-mode PDT/PTT for more efficient treatment of B16F0 melanoma tumors than that of doxorubicin, a clinically used drug.
Another group found that singlet oxygen could be produced when irradiated with a wide range of wavelengths (660–975 nm) [64]. Even under low-intensity light irradiation of 200 mW/cm2, the highest production of 1O2 was observed when a wavelength overlapped with the localized surface plasmon resonance (LSPR) peak, which is a characteristic of gold nanoparticles.
Many previous studies have demonstrated the ability of metal nanoparticles to efficiently excite PS through a single-photon excitation mechanism to generate singlet oxygen, which has been applied to typical PDT therapy [65][66]. However, one-photon excitation can cause potential photodamage to tissues adjacent to the tumor site due to the high energy provided by the comparatively short light wavelength. Therefore, two-photon excitation that precisely manipulates the therapeutic dose is preferable in this sense. To overcome this, a two-photon PDT was developed using a femtosecond laser beam capable of obtaining a high luminous flux. In one study, two-photon-induced singlet oxygen generation was observed by irradiating femtosecond laser pulses at 800 nm to aggregates of gold nanospheres and gold nanorods developed using non-agglomerated or aggregated gold nanoparticles [67]. As a result, the 1O2 generation capacity in gold nanoparticle was generally enhanced by the agglomerated state and was 8.3 times higher than that of the non-agglomerated gold nanoparticles. A similar trend was observed when the agglomerated gold nanorods were used; the singlet oxygen production efficiency was improved by 1.8 times compared to the non-agglomerated gold nanorods.
With the rapid advances in nanotechnology, there are a variety of synthetic methods available to researchers to obtain gold nanoparticles with suitable structures and features for PDT applications [68]. In addition to the various physicochemical properties, the additional chemical modification potential mentioned above could improve bioavailability and usability, suggesting gold nanoparticles as a promising candidate for clinical cancer treatment.
2.3.3. Graphene Nanomaterials
Graphene-based nanomaterials, including graphene oxide (GO) and graphene quantum dots (GQD), have been widely used for cancer treatment such as anticancer drug delivery and PDT [69][70][71]. GO produced through oxidation process shows more favorable properties in terms of PS transport mediation due to improved water solubility and various functionalization chemistries. Characterized by abundant oxygen-containing moieties on their surface, GO nanomaterials allow further modification by many functional molecules such as targeting agents, activators and hydrophilic macromolecules, expanding biological applications and reducing toxicity [72]. Because the fluorescence quenching ability of GO nanomaterials is very high, it modulates the activity that generates ROS, further expanding the applications of PDT (Figure 4).
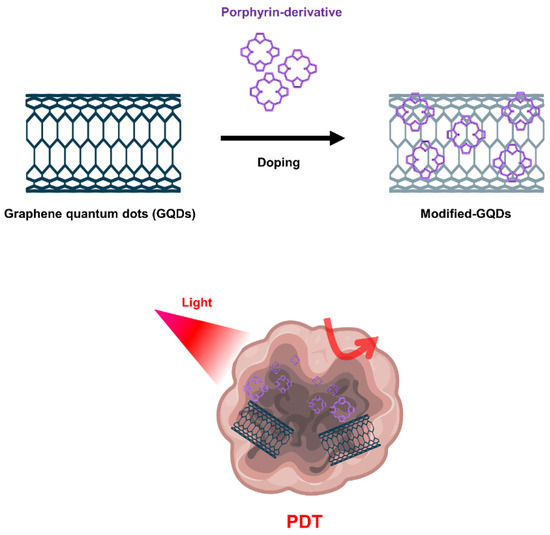
Figure 4. Graphene quantum dots (GQDs)-based nanomaterials for PDT.
Numerous studies have been conducted to achieve tumor targeting, in vivo imaging, and improved PDT effects through functionalization on the GO surface. In one study, PEG-functionalized GO was loaded with the PS 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-alpha (HPPH) via supramolecular π-π stacking [73]. HPPH radiolabeled with 64Cu enabled in vivo positron emission tomography and fluorescence imaging, resulting in improved cellular uptake of HPPH compared to free HPPH with GO-PEG-HPPH through a more aggressive endocytosis strategy. As a result, GO-PEG-HPPH exhibited enhanced phototoxicity to breast cancer cells when irradiated with light at a wavelength of 671 nm. Through in vivo experiments, mice injected with GO-PEG-HPPH showed a 16-day longer lifespan than mice treated with free HPPH. This indicates that GO-PEG-HPPH utilizing GO as a nanocarrier delivered the drug more efficiently and thereby increased long-term survival. In another study, the PS hypocrelin A (HA) and TiO2 nanoparticles were mounted on GO surfaces to form a light-sensitive drug delivery system [74]. By loading TiO2 onto GO, ROS could be generated upon exposure to visible light, and the ability to generate ROS was improved through a mutual sensitization mechanism in which a sensitizing effect contributed by the HA-TiO2 stable complex. The generated ROS were able to destroy GO, indicating a potential use of this drug delivery system in clinical PDT in terms of metabolism.
In another study, PS Ce6 was conjugated to GO via a redox-responsive cleavable disulfide linker (GO-SS-Ce6) to develop a form that could be released on-demand from cancer cells at significantly higher GSH concentrations compared to normal cells. Therefore, fluorescence and ROS generation were selectively activated by redox agents such as glutathione at high concentrations in tumor cells [75]. On the other hand, in the absence of glutathione, the fluorescence of Ce6 bound to GO was largely quenched due to the FRET process, avoiding the nonspecific excitation and poor targeting ability of PS. The developed GO-SS-Ce6 complex has been proposed as an effective drug delivery vehicle with the strengths of GO’s high surface area and improved chemical tethering properties.
Furthermore, GQDs doped with quantum dots in graphene could provide excellent quantum yield of singlet oxygen as a PDT agent [76]. It is known as a common method to synthesize GQDs using polythiophene as a carbon precursor using hydrothermal methods. The GQDs fabricated in the study were excited by visible light and showed photodynamic activity; their PDT effects were observed through apoptosis of HeLa cells and oncolysis of BALB/nude mice with breast cancer. On the other hand, more advanced studies showed that GQDs could be functionalized and doped with nitrogen and amino groups to show that the amino-N-GQDs exhibited excellent singlet oxygen generation capacity in the NIR region (800 nm) [77].
2.3.4. Upconversion Nanoparticles
Upconversion nanoparticles (UCNPs) are a unique class of optical nanomaterials characterized by their ability to convert low-energy NIR light into high-energy visible/ultraviolet light using a nonlinear anti-Stokes mechanism [78]. The upconversion phenomenon is based on inorganic host crystal lattices doped with trivalent lanthanide ions such as Yb3+, Er3+, and Tm3+. UCNPs require the presence of two different dopant ions [79]. One acts as a sensitizer to absorb NIR radiation, and the other acts as an activator to emit visible light. Two frequently used rare earth ion pairs are ytterbium-thulium (Yb3+-Tm3+) and ytterbium-erbium (Yb3+-Er3+). The Yb3+ ions act as antennas, absorbing NIR light at about 900–1100 nm and transmitting it to the lanthanide ions, where they mutually upconvert. If this ion is Er3+, green and red emission is observed, whereas if it is Tm3+, the emitted light is near-ultraviolet, blue and red. In addition, the emission band of UCNP is similar to the band in which PS can be excited, which is characterized by improved ROS production efficiency [53]. In this regard, UCNP may serve as a promising carrier to overcome the limitations of PDT due to the insufficient tissue penetrating ability of short wavelengths (600–850 nm) (Figure 5).
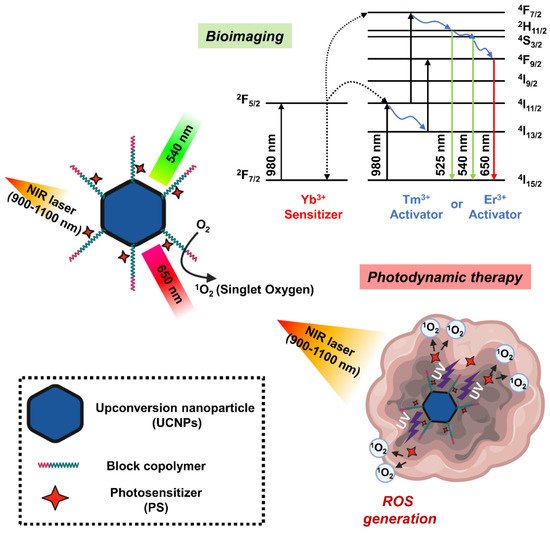
Figure 5. Schematic diagram showing the mechanism of photodynamic therapy and bioimaging through long-wavelength to short-wavelength conversion of upconversion nanoparticles (UCNPs).
The NaYF4: Yb3+/ Er3+, the first UCNPs used in PDT studies, showed strong emission spectrum in the visible region around 537 and 635 nm when excited by an infrared light source of 974 nm [80]. During the silica coating procedure in the UNCP synthesis, the PS molecule merocyanine 540 (MC-540) was mounted on the nanoparticle. However, the activation wavelength of these PSs is under 700 nm, which is a range in which endogenous molecules such as hemoglobin have strong absorption, a great limitation in their use in PDT. A study successfully detected the generation of singlet oxygen mediated by UCNPs coated with MC-540 with NIR excitation by measuring the decrease in the fluorescence band of the 1O2 sensor 9,10-anthracenedipropionic acid. Moreover, the first application of UCNP-mediated PDT for in vivo tumor therapy is NaYF4:Yb/Er nanoparticles coated with mesoporous silica as nano-transducers and carriers of two different PSs such as MC-540 and ZnPc [81]. Another study found that UCNPs synthesized using dual PS had higher PDT efficacy than using single PS, with improved ROS production capacity and enhanced cytotoxicity. In the tumor-bearing mice, both intratumoral injection of UCNP or intravenous injection of FA and PEG-modified UCNPs (FA-PEG-UCNP) into tumor resulted in tumor growth inhibition at 980 nm excitation. In addition, the tumor-targeting ability and circulating lifespan of UCNP were improved by FA and PEG, respectively, indicating a greater PDT effect when administered intravenously.
One research team prepared NaYF4:Er/Yb/Gd upconversion nanocrystals by doping NaYF4:Yb/Er UCNP with gadolinium ions and loading them with PS drugs to use as a carrier [82]. Through a water-in-oil inverse microemulsion strategy, methylene blue (MB), a hydrophilic PS drug, was efficiently conjugated to UCNPs in a silica matrix to provide UCNP/MB nanocomposites with a particle size less than 50 nm. The obtained UCNP/MB-based PDT drug successfully generated singlet oxygen at 980 nm excitation, whereas no signal was observed with free MB solution alone or with NaYF4:Er/Yb/Gd under the same conditions. Furthermore, polymer-coated NaYF4:Yb/Er nanoparticles were used as transport mediators of PS Ce6 to form UCNP-Ce6 supramolecular complexes [83]. Because this UCNP-Ce6 nanosystem showed two emission bands at 550 nm and 660 nm with 980 nm irradiation, PDT performance was improved in that the 660 nm emission wavelength overlapped the absorption band of Ce6, and singlet oxygen production was increased under NIR light irradiation. In particular, there were few observations of UCNPs administered to mice after 1–2 months, demonstrating their nontoxicity to the treated animals.
Although it is common to form NaYF4 crystals with a host co-doped with Yb3+/Er3+ in UCNP-based PDT, doping NaYF4 with a Yb3+/Tm3+ couple shows a similar phenomenon. In one study, NaYF4:Yb/Tm UCNPs were coated with a nanometer silica layer, which was further modified with (3-aminopropyl)triethoxysilane APTES using the Stöber method [84]. After that, the UCNPs were covalently bound to PS Ce6 via the amino group of the silica layer. A low concentration (50 μg/mL) of this UCNP-Ce6 nanocomposite was used to kill 50% of CF-7 human breast adenocarcinoma cells at a low dose (7 mW/cm2) of 980 nm light for 10 min. Furthermore, they achieved a cell viability greater than 90% under the same conditions without light irradiation, indicating low toxicity of this UCNP-Ce6 nanosystem in the effective concentration range. Alternatively, LiYF4:Tm3+/Yb3+-UCNPs prepared using m-THPC with PS modified with 4-(bromomethyl)benzoic acid performed better when activated with 980 nm NIR irradiation compared to conventional NaYF4UCNPs. They emitted an intense blue color and produced a larger amount of singlet oxygen [49][85][86].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10010096
References
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood-Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834.
- Chaichana, K.L.; Pinheiro, L.; Brem, H. Delivery of local therapeutics to the brain: Working toward advancing treatment for malignant gliomas. Ther. Deliv. 2015, 6, 353–369.
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628.
- Abbasi, J. Guided Ultrasound Opens Blood-Brain Barrier to Cancer Drugs. JAMA 2021, 326, 1785.
- Lombardo, S.M.; Schneider, M.; Tureli, A.E.; Gunday Tureli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883.
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdeel, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci. Rep. 2019, 9, 8278.
- Del Amo, L.; Cano, A.; Ettcheto, M.; Souto, E.B.; Espina, M.; Camins, A.; Garcia, M.L.; Sanchez-Lopez, E. Surface Functionalization of PLGA Nanoparticles to Increase Transport across the BBB for Alzheimer’s Disease. Appl. Sci. 2021, 11, 4305.
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 2015, 11, 1603–1611.
- Calixto, G.M.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342.
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632.
- Garg, T.; Jain, N.K.; Rath, G.; Goyal, A.K. Nanotechnology-Based Photodynamic Therapy: Concepts, Advances, and Perspectives. Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 389–439.
- Uthaman, S.; Pillarisetti, S.; Mathew, A.P.; Kim, Y.; Bae, W.K.; Huh, K.M.; Park, I.K. Long circulating photoactivable nanomicelles with tumor localized activation and ROS triggered self-accelerating drug release for enhanced locoregional chemo-photodynamic therapy. Biomaterials 2020, 232, 119702.
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. A pH-sensitive micelle composed of heparin, phospholipids, and histidine as the carrier of photosensitizers: Application to enhance photodynamic therapy of cancer. Int. J. Biol. Macromol. 2017, 98, 125–138.
- Kim, D.H.; Hwang, H.S.; Na, K. Photoresponsive Micelle-Incorporated Doxorubicin for Chemo-Photodynamic Therapy to Achieve Synergistic Antitumor Effects. Biomacromolecules 2018, 19, 3301–3310.
- Li, R.; Shan, L.; Yao, Y.; Peng, F.; Jiang, S.; Yang, D.; Ling, G.; Zhang, P. Black phosphorus nanosheets and docetaxel micelles co-incorporated thermoreversible hydrogel for combination chemo-photodynamic therapy. Drug Deliv. Transl. Res. 2021, 11, 1133–1143.
- Wu, J.; Xia, L.; Liu, Z.; Xu, Z.; Cao, H.; Zhang, W. Fabrication of a Dual-Stimuli-Responsive Supramolecular Micelle from a Pillararene-Based Supramolecular Diblock Copolymer for Photodynamic Therapy. Macromol. Rapid Commun. 2019, 40, e1900240.
- Kim, Y.J.; Lee, H.I.; Kim, J.K.; Kim, C.H.; Kim, Y.J. Peptide 18-4/chlorin e6-conjugated polyhedral oligomeric silsesquioxane nanoparticles for targeted photodynamic therapy of breast cancer. Colloids Surf. B Biointerfaces 2020, 189, 110829.
- Um, W.; Park, J.; Ko, H.; Lim, S.; Yoon, H.Y.; Shim, M.K.; Lee, S.; Ko, Y.J.; Kim, M.J.; Park, J.H.; et al. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-DEVD-anticancer drug conjugate for targeted cancer therapy. Biomaterials 2019, 224, 119494.
- Lu, L.; Zhao, X.J.; Fu, T.W.; Li, K.; He, Y.; Luo, Z.; Dai, L.L.; Zeng, R.; Cai, K.Y. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666.
- Li, L.; Cho, H.; Kim, S.; Kang, H.C.; Huh, K.M. Polyelectrolyte nanocomplex formation of heparin-photosensitizer conjugate with polymeric scavenger for photodynamic therapy. Carbohydr. Polym. 2015, 121, 122–131.
- Wu, Y.; Li, F.; Zhang, X.; Li, Z.; Zhang, Q.; Wang, W.; Pan, D.; Zheng, X.; Gu, Z.; Zhang, H.; et al. Tumor microenvironment-responsive PEGylated heparin-pyropheophorbide-a nanoconjugates for photodynamic therapy. Carbohydr. Polym. 2021, 255, 117490.
- Wang, M.; Geilich, B.M.; Keidar, M.; Webster, T.J. Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. Int. J. Nanomed. 2017, 12, 4117–4127.
- Cho, M.H.; Li, Y.; Lo, P.C.; Lee, H.; Choi, Y. Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer. Nano-Micro Lett. 2020, 12, 47.
- Pan, Y.T.; Ding, Y.F.; Han, Z.H.; Yuwen, L.; Ye, Z.; Mok, G.S.P.; Li, S.; Wang, L.H. Hyaluronic acid-based nanogels derived from multicomponent self-assembly for imaging-guided chemo-photodynamic cancer therapy. Carbohydr. Polym. 2021, 268, 118257.
- Chang, E.; Bu, J.; Ding, L.; Lou, J.W.H.; Valic, M.S.; Cheng, M.H.Y.; Rosilio, V.; Chen, J.; Zheng, G. Porphyrin-lipid stabilized paclitaxel nanoemulsion for combined photodynamic therapy and chemotherapy. J. Nanobiotechnol. 2021, 19, 154.
- Sundaram, P.; Abrahamse, H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 4745.
- Zhou, Y.; Chang, C.; Liu, Z.; Zhao, Q.; Xu, Q.; Li, C.; Chen, Y.; Zhang, Y.; Lu, B. Hyaluronic Acid-Functionalized Hollow Mesoporous Silica Nanoparticles as pH-Sensitive Nanocarriers for Cancer Chemo-Photodynamic Therapy. Langmuir 2021, 37, 2619–2628.
- Potara, M.; Nagy-Simon, T.; Focsan, M.; Licarete, E.; Soritau, O.; Vulpoi, A.; Astilean, S. Folate-targeted Pluronic-chitosan nanocapsules loaded with IR780 for near-infrared fluorescence imaging and photothermal-photodynamic therapy of ovarian cancer. Colloids Surf. B Biointerfaces 2021, 203, 111755.
- Wang, X.; Li, S.; Liu, H. Co-delivery of chitosan nanoparticles of 5-aminolevulinic acid and shGBAS for improving photodynamic therapy efficacy in oral squamous cell carcinomas. Photodiagn. Photodyn. Ther. 2021, 34, 102218.
- Zhu, T.; Shi, L.; Ma, C.; Xu, L.; Yang, J.; Zhou, G.; Zhu, X.; Shen, L. Fluorinated chitosan-mediated intracellular catalase delivery for enhanced photodynamic therapy of oral cancer. Biomater. Sci. 2021, 9, 658–662.
- Gaio, E.; Conte, C.; Esposito, D.; Miotto, G.; Quaglia, F.; Moret, F.; Reddi, E. Co-delivery of Docetaxel and Disulfonate Tetraphenyl Chlorin in One Nanoparticle Produces Strong Synergism between Chemo- and Photodynamic Therapy in Drug-Sensitive and -Resistant Cancer Cells. Mol. Pharm. 2018, 15, 4599–4611.
- Li, W.; Peng, J.; Tan, L.; Wu, J.; Shi, K.; Qu, Y.; Wei, X.; Qian, Z. Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by Lyp-1 modified Docetaxel/IR820 Co-loaded micelles. Biomaterials 2016, 106, 119–133.
- Zhou, S.Y.; Hu, X.L.; Xia, R.; Liu, S.; Pei, Q.; Chen, G.; Xie, Z.G.; Jing, X.B. A Paclitaxel Prodrug Activatable by Irradiation in a Hypoxic Microenvironment. Angew. Chem. Int. Ed. 2020, 59, 23198–23205.
- Pan, Q.Q.; Tian, J.J.; Zhu, H.H.; Hong, L.J.; Mao, Z.W.; Oliveira, J.M.; Reis, R.L.; Li, X. Tumor-Targeting Polycaprolactone Nanoparticles with Codelivery of Paclitaxel and IR780 for Combinational Therapy of Drug-Resistant Ovarian Cancer. ACS Biomater. Sci. Eng. 2020, 6, 2175–2185.
- Wang, D.; Zhang, S.; Zhang, T.; Wan, G.; Chen, B.; Xiong, Q.; Zhang, J.; Zhang, W.; Wang, Y. Pullulan-coated phospholipid and Pluronic F68 complex nanoparticles for carrying IR780 and paclitaxel to treat hepatocellular carcinoma by combining photothermal therapy/photodynamic therapy and chemotherapy. Int. J. Nanomed. 2017, 12, 8649–8670.
- Yang, X.; Shi, X.; Zhang, Y.; Xu, J.; Ji, J.; Ye, L.; Yi, F.; Zhai, G. Photo-triggered self-destructive ROS-responsive nanoparticles of high paclitaxel/chlorin e6 co-loading capacity for synergetic chemo-photodynamic therapy. J. Control. Release 2020, 323, 333–349.
- de Paula Rodrigues, R.; Tini, I.R.; Soares, C.P.; da Silva, N.S. Effect of photodynamic therapy supplemented with quercetin in HEp-2 cells. Cell Biol. Int. 2014, 38, 716–722.
- Thakur, N.S.; Mandal, N.; Patel, G.; Kirar, S.; Reddy, Y.N.; Kushwah, V.; Jain, S.; Kalia, Y.N.; Bhaumik, J.; Banerjee, U.C. Co-administration of zinc phthalocyanine and quercetin via hybrid nanoparticles for augmented photodynamic therapy. Nanomedicine 2021, 33, 102368.
- He, J.; Huang, X.; Li, Y.C.; Liu, Y.; Babu, T.; Aronova, M.A.; Wang, S.; Lu, Z.; Chen, X.; Nie, Z. Self-assembly of amphiphilic plasmonic micelle-like nanoparticles in selective solvents. J. Am. Chem. Soc. 2013, 135, 7974–7984.
- Huntosova, V.; Datta, S.; Lenkavska, L.; Macajova, M.; Bilcik, B.; Kundekova, B.; Cavarga, I.; Kronek, J.; Jutkova, A.; Miskovsky, P.; et al. Alkyl Chain Length in Poly(2-oxazoline)-Based Amphiphilic Gradient Copolymers Regulates the Delivery of Hydrophobic Molecules: A Case of the Biodistribution and the Photodynamic Activity of the Photosensitizer Hypericin. Biomacromolecules 2021, 22, 4199–4216.
- Li, H.; Yu, Z.; Wang, S.; Long, X.; Zhang, L.M.; Zhu, Z.; Yang, L. Photosensitizer-encapsulated amphiphilic chitosan derivative micelles: Photoactivity and enhancement of phototoxicity against human pancreatic cancer cells. J. Photochem. Photobiol. B Biol. 2015, 142, 212–219.
- Bazylinska, U.; Kulbacka, J.; Chodaczek, G. Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment. Pharmaceutics 2019, 11, 405.
- Malacarne, M.C.; Banfi, S.; Rugiero, M.; Caruso, E. Drug delivery systems for the photodynamic application of two photosensitizers belonging to the porphyrin family. Photochem. Photobiol. Sci. 2021, 20, 1011–1025.
- Xu, L.; Zhang, W.; Cai, H.; Liu, F.; Wang, Y.; Gao, Y.; Zhang, W. Photocontrollable release and enhancement of photodynamic therapy based on host-guest supramolecular amphiphiles. J. Mater. Chem. B 2015, 3, 7417–7426.
- Elschner, T.; Wondraczek, H.; Heinze, T. Syntheses and detailed structure characterization of dextran carbonates. Carbohydr. Polym. 2013, 93, 216–223.
- Yang, C.; Fu, Y.; Huang, C.; Hu, D.; Zhou, K.; Hao, Y.; Chu, B.; Yang, Y.; Qian, Z. Chlorin e6 and CRISPR-Cas9 dual-loading system with deep penetration for a synergistic tumoral photodynamic-immunotherapy. Biomaterials 2020, 255, 120194.
- Yang, X.; Cai, X.; Yu, A.; Xi, Y.; Zhai, G. Redox-sensitive self-assembled nanoparticles based on alpha-tocopherol succinate-modified heparin for intracellular delivery of paclitaxel. J. Colloid Interface Sci. 2017, 496, 311–326.
- Jena, S.K.; Sangamwar, A.T. Polymeric micelles of amphiphilic graft copolymer of alpha-tocopherol succinate-g-carboxymethyl chitosan for tamoxifen delivery: Synthesis, characterization and in vivo pharmacokinetic study. Carbohydr. Polym. 2016, 151, 1162–1174.
- Wang, Y.H.; Song, S.Y.; Zhang, S.T.; Zhang, H.J. Stimuli-responsive nanotheranostics based on lanthanide-doped upconversion nanoparticles for cancer imaging and therapy: Current advances and future challenges. Nano Today 2019, 25, 38–67.
- Pandya, A.D.; Overbye, A.; Sahariah, P.; Gaware, V.S.; Hogset, H.; Masson, M.; Hogset, A.; Maelandsmo, G.M.; Skotland, T.; Sandvig, K.; et al. Drug-Loaded Photosensitizer-Chitosan Nanoparticles for Combinatorial Chemo- and Photodynamic-Therapy of Cancer. Biomacromolecules 2020, 21, 1489–1498.
- Xu, X.; Chong, Y.; Liu, X.; Fu, H.; Yu, C.; Huang, J.; Zhang, Z. Multifunctional nanotheranostic gold nanocages for photoacoustic imaging guided radio/photodynamic/photothermal synergistic therapy. Acta Biomater. 2019, 84, 328–338.
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. A dual-targeted hyaluronic acid-gold nanorod platform with triple-stimuli responsiveness for photodynamic/photothermal therapy of breast cancer. Acta Biomater. 2019, 83, 400–413.
- Krajczewski, J.; Rucinska, K.; Townley, H.E.; Kudelski, A. Role of various nanoparticles in photodynamic therapy and detection methods of singlet oxygen. Photodiagn. Photodyn. Ther. 2019, 26, 162–178.
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Ghosh, S.; Chatterjee, S.; Das, J.; Brahmachari, G.; Sil, P.C. In vivo therapeutic evaluation of a novel bis-lawsone derivative against tumor following delivery using mesoporous silica nanoparticle based redox-responsive drug delivery system. Mater. Sci. Eng. C 2021, 126.
- Chen, H.; Kuang, Y.; Liu, R.; Chen, Z.Y.; Jiang, B.B.; Sun, Z.G.; Chen, X.Q.; Li, C. Dual-pH-sensitive mesoporous silica nanoparticle-based drug delivery system for tumor-triggered intracellular drug release. J. Mater. Sci. 2018, 53, 10653–10665.
- Han, R.L.; Wu, S.; Yan, Y.Y.; Chen, W.; Tang, K.Q. Construction of ferrocene modified and indocyanine green loaded multifunctional mesoporous silica nanoparticle for simultaneous chemodynamic/photothermal/photodynamic therapy. Mater. Today Commun. 2021, 26, 101842.
- Kim, J.; Cho, H.R.; Jeon, H.; Kim, D.; Song, C.; Lee, N.; Choi, S.H.; Hyeon, T. Continuous O-2-Evolving MnFe2O4 Nanoparticle-Anchored Mesoporous Silica Nanoparticles for Efficient Photodynamic Therapy in Hypoxic Cancer. J. Am. Chem. Soc. 2017, 139, 10992–10995.
- Sun, J.; Fan, Y.; Zhang, P.; Zhang, X.; Zhou, Q.; Zhao, J.; Ren, L.Q. Self-enriched mesoporous silica nanoparticle composite membrane with remarkable photodynamic antimicrobial performances. J. Colloid Interfaces Sci. 2020, 559, 197–205.
- Bharathiraja, S.; Moorthy, M.S.; Manivasagan, P.; Seo, H.; Lee, K.D.; Oh, J. Chlorin e6 conjugated silica nanoparticles for targeted and effective photodynamic therapy. Photodiagn. Photodyn. Ther. 2017, 19, 212–220.
- Huang, C.L.; Zhang, Z.M.; Guo, Q.; Zhang, L.; Fan, F.; Qin, Y.; Wang, H.; Zhou, S.; Ou, W.B.Y.; Sun, H.F.; et al. A Dual-Model Imaging Theragnostic System Based on Mesoporous Silica Nanoparticles for Enhanced Cancer Phototherapy. Adv. Healthc. Mater. 2019, 8, e1900840.
- Dey, P.; Blakey, I.; Stone, N. Diagnostic prospects and preclinical development of optical technologies using gold nanostructure contrast agents to boost endogenous tissue contrast. Chem. Sci. 2020, 11, 8671–8685.
- Vankayala, R.; Huang, Y.K.; Kalluru, P.; Chiang, C.S.; Hwang, K.C. First Demonstration of Gold Nanorods-Mediated Photodynamic Therapeutic Destruction of Tumors via Near Infra-Red Light Activation. Small 2014, 10, 1612–1622.
- Vankayala, R.; Lin, C.C.; Kalluru, P.; Chiang, C.S.; Hwang, K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014, 35, 5527–5538.
- Lv, J.L.; Zhang, X.; Li, N.N.; Wang, B.J.; He, S.L. Absorption-dependent generation of singlet oxygen from gold bipyramids excited under low power density. RSC Adv. 2015, 5, 81897–81904.
- Vankayala, R.; Sagadevan, A.; Vijayaraghavan, P.; Kuo, C.L.; Hwang, K.C. Metal Nanoparticles Sensitize the Formation of Singlet Oxygen. Angew. Chem. Int. Ed. 2011, 50, 10640–10644.
- Pasparakis, G. Light-Induced Generation of Singlet Oxygen by Naked Gold Nanoparticles and its Implications to Cancer Cell Phototherapy. Small 2013, 9, 4130–4134.
- Jiang, C.F.; Zhao, T.T.; Yuan, P.Y.; Gao, N.Y.; Pan, Y.L.; Guan, Z.P.; Zhou, N.; Xu, Q.H. Two-Photon Induced Photoluminescence and Singlet Oxygen Generation from Aggregated Gold Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4972–4977.
- Pakravan, A.; Salehi, R.; Mahkam, M. Comparison study on the effect of gold nanoparticles shape in the forms of star, hallow, cage, rods, and Si -Au and Fe -Au core-shell on photothermal cancer treatment. Photodiagn. Photodyn. Ther. 2021, 33, 102144.
- Shih, C.Y.; Huang, W.L.; Chiang, I.T.; Su, W.C.; Teng, H.S. Biocompatible hole scavenger-assisted graphene oxide dots for photodynamic cancer therapy. Nanoscale 2021, 13, 8431–8441.
- Mangalath, S.; Babu, P.S.S.; Nair, R.R.; Manu, P.M.; Krishna, S.; Nair, S.A.; Joseph, J. Graphene Quantum Dots Decorated with Boron Dipyrromethene Dye Derivatives for Photodynamic Therapy. ACS Appl. Nano Mater. 2021, 4, 4162–4171.
- Roeinfard, M.; Zahedifar, M.; Darroudi, M.; Zak, A.K.; Sadeghi, E. Preparation and characterization of selenium-decorated graphene quantum dots with high afterglow for application in photodynamic therapy. Luminescence 2020, 35, 891–896.
- Yi, L.Y.; Zhang, Y.N.; Shi, X.Q.; Du, X.Y.; Wang, X.Y.; Yu, A.H.; Zhai, G.X. Recent progress of functionalised graphene oxide in cancer therapy. J. Drug Target. 2019, 27, 125–144.
- Rong, P.F.; Yang, K.; Srivastan, A.; Kiesewetter, D.O.; Yue, X.Y.; Wang, F.; Nie, L.M.; Bhirde, A.; Wang, Z.; Liu, Z.; et al. Photosensitizer Loaded Nano-Graphene for Multimodality Imaging Guided Tumor Photodynamic Therapy. Theranostics 2014, 4, 229–239.
- Ding, Y.; Zhou, L.; Chen, X.; Wu, Q.; Song, Z.Y.; Wei, S.H.; Zhou, J.H.; Shen, J. Mutual sensitization mechanism and self-degradation property of drug delivery system for in vitro photodynamic therapy. Int. J. Pharmaceut. 2016, 498, 335–346.
- Cho, Y.; Choi, Y. Graphene oxide-photosensitizer conjugate as a redox-responsive theranostic agent. Chem. Commun. 2012, 48, 9912–9914.
- Ge, J.C.; Lan, M.H.; Zhou, B.J.; Liu, W.M.; Guo, L.; Wang, H.; Jia, Q.Y.; Niu, G.L.; Huang, X.; Zhou, H.Y.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 1–8.
- Kuo, W.S.; Shao, Y.T.; Huang, K.S.; Chou, T.M.; Yang, C.H. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446.
- Fang, W.K.; Wei, Y.C. Upconversion nanoparticle as a theranostic agent for tumor imaging and therapy. J. Innov. Opt. Health Sci. 2016, 9, 1630006.
- Hamblin, M.R. Upconversion in photodynamic therapy: Plumbing the depths. Dalton Trans. 2018, 47, 8571–8580.
- Zhang, P.; Steelant, W.; Kumar, M.; Scholfield, M. Versatile photosensitizers for photodynamic therapy at infrared excitation. J. Am. Chem. Soc. 2007, 129, 4526–4527.
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585.
- Chen, F.; Zhang, S.J.; Bu, W.B.; Chen, Y.; Xiao, Q.F.; Liu, J.A.; Xing, H.Y.; Zhou, L.P.; Peng, W.J.; Shi, J.L. A Uniform Sub-50 nm-Sized Magnetic/Upconversion Fluorescent Bimodal Imaging Agent Capable of Generating Singlet Oxygen by Using a 980 nm Laser. Chem. Eur. J. 2012, 18, 7082–7090.
- Wang, C.; Tao, H.Q.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154.
- Dou, Q.Q.; Teng, C.P.; Ye, E.Y.; Loh, X.J. Effective near-infrared photodynamic therapy assisted by upconversion nanoparticles conjugated with photosensitizers. Int. J. Nanomed. 2015, 10, 419–432.
- Yu, Q.; Rodriguez, E.M.; Naccache, R.; Forgione, P.; Lamoureux, G.; Sanz-Rodriguez, F.; Scheglmann, D.; Capobianco, J.A. Chemical modification of temoporfin—A second generation photosensitizer activated using upconverting nanoparticles for singlet oxygen generation. Chem. Commun. 2014, 50, 12150–12153.
- Li, K.M.; Hong, E.L.; Wang, B.; Wang, Z.Y.; Zhang, L.W.; Hu, R.X.; Wang, B.Q. Advances in the application of upconversion nanoparticles for detecting and treating cancers. Photodiagn. Photodyn. Ther. 2019, 25, 177–192.
This entry is offline, you can click here to edit this entry!
