The microbiome, as a community of microorganisms and their structural elements, genomes, metabolites/signal molecules, has been shown to play an important role in human health, with significant beneficial applications for gut health. Skin microbiome has emerged as a new field with high potential to develop disruptive solutions to manage skin health and disease. Despite an incomplete toolbox for skin microbiome analyses, much progress has been made towards functional dissection of microbiomes and host-microbiome interactions. A standardized and robust investigation of the skin microbiome is necessary to provide accurate microbial information and set the base for a successful translation of innovations in the dermo-cosmetic field.
1. Introduction
Applications of Microbiome sciences are very large and have been proposed as a potential target solution for the 21st century socio-economic and environmental challenges [
1]. For several decades, scientists have been interested in the microbiome and its impact on human health. A major focus was put on the gut microbiome, and its role in human health has been well established [
2]. New knowledge on lung, oral cavity, and skin microbiome is beginning to emerge [
3]. A deeper knowledge of the microbiome, specifically that of the skin, opens perspectives for a revolution in dermo-cosmetic development. These recent discoveries have changed our perception of the role of bacteria in skin health. For example, microbiome-derived and personalized dermo-cosmetic development would be possible, due to the advancement in skin microbiome analysis and diagnosis [
4]. New products that respect, protect or rebalance the skin microbiome are a new trend in the dermo-cosmetic industry.
2. Microbiome-Based Cosmetic Solutions and Technologies
Many of the skin conditions are multifactorial, however, the microbiome is a key factor in skin disorders. The interplay between the microbiome and the skin is key for its homeostasis health. Intervening and finely modulating the microbiome to correct skin conditions described above is a rising field of research. These interventions are mainly realized by prebiotics, postbiotics, and probiotics, as well as microbiota transplant. The latter is still in its infancy phase for the skin. In cosmetic/dermatology applications, a focus concentrates on the first three paths.
The microbiome has been extensively studied and reported in the field of nutrition [
199]. Although some definitions exist on the World Health Organization level, there are currently no available international guidelines regarding the definitions or terminologies applicable for cosmetic ingredients that work with the skin’s microbiome. Current definitions consider probiotics to be living microorganisms that must be ingested in a sufficient amount to have a positive effect on health that is not limited to the nutritional effects alone [
200,
201,
202].
Prebiotics are a food ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon the host’s health [
199].
Very recently, the International Scientific Association of Probiotics and Prebiotics (ISAPP) defined the scope of postbiotics as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host”.
Postbiotics could be intentionally inactivated microbial cells with or without metabolites, or cell components that contribute to establishing host health benefits.
The gut is not the only site of action of postbiotics. They could also be administered on a host surface, such as in the oral cavity or on the skin [
203].
The topic of the cosmetic microbiome was taken up in 2018 by the International Cooperation on Cosmetics Regulation (ICCR), a voluntary international group of cosmetic regulatory authorities and cosmetic industry trade associations from Brazil, Canada, Chinese Taipei, the European Union, Japan, the Republic of Korea, and the United States. They considered that new technologies exploring the relationship between the human microbiome and healthy skin were an area of increasing interest and the safety, quality, regulation, and potential development of international guidelines for products arising from these technologies would be a worthwhile topic for the ICCR.
In 2020 they developed a set of categories and descriptors that could be used to group and categorize microbiome-related products, their ingredients, and other relevant approaches, in a cosmetic/skin-relevant context [
204].
These ingredients were divided into two main categories based on viability: viable (live or dormant)—encompassing only probiotics (based on biological origin), and non-viable ingredients. The non-viable ingredients were further divided into two sub-categories; prebiotics (by their intended action on the skin microbiota) and postbiotics (based on their biological origin) (Table 2).
Table 2. Resuming the cosmetic description of prebiotic and postbiotic ingredients.
| Postbiotic (Including Probiotic Fraction or Extract) |
Prebiotic |
Non-viable ingredients comprised of inactivated microorganisms and/or soluble factors (products or metabolic by-products) released by live or inactivated microorganisms, added to a cosmetic product to achieve a cosmetic benefit at the application site, either directly or via an effect on the existing microbiota.
Categories: 1/Ferments, lysates, extracts, filtrates, 2/Non-viable microorganisms (inactivated/heat-killed), 3/Metabolic products/by-products (isolated) |
Non-viable ingredients are added to a cosmetic product to be actively used as nutrients by the microbiota of the application site to achieve a cosmetic benefit.
Examples: ingredients such as fibers, sugars, minerals, but also complex biological mixtures/extracts, etc. |
Postbiotic products/ingredients belong to the non-viable category. Based on their biological origin, postbiotic ingredients (ferments, extracts, lysates, filtrates) share a common description: “
Non-viable ingredients comprised of inactivated microorganisms and/or soluble factors (products or metabolic by-products) released by live or inactivated microorganisms, added to a cosmetic product to achieve a cosmetic benefit at the application site, either directly or via
an effect on the existing microbiota” [
204].
In cosmetics, postbiotics may be an alternative to the use of live whole microorganisms in probiotic form. To summarize the product entries, postbiotic ingredients were divided into three types:
“Ferments, lysates, extracts, filtrates or any combination of these ingredients that are not living but which have been obtained by means of probiotic bacteria (Bacillus, Bifidobacterium, Lactobacillus, Lactococcus, Vitreoscilla, Streptococcus thermophilus, Leuconostoc) or fungi used primarily as fermentation facilitators (Saccharomyces, Candida bombicola, Kloeckera, Hansenula-Pichia, Aspergillus)”:
“Non-viable microorganisms (inactivated/heat-killed), mostly lactic-acid forming bacteria: Enterococcus faecalis, Lactobacillus (paracasei, casei, acidophilus), Lactococcus, or Vitreoscilla filiform”.
“Metabolic products/by-products (isolated) including bacteriocin extract, ectoin, succinic acid, lactic acid, hydrolyzed yogurt protein, sodium hyaluronate, and milk proteins” [
204].
3. Future Implications/Outlook
Postbiotics, including probiotic fractions and effector molecules, are solutions already used in the dermo-cosmetic field; however, the ambition for the cosmetic industry is to add live probiotics to cosmetic formulas with the expectation of potentially higher performance that would probably be driven by a dialogue between added living-microbes and host cells.
However, the use of probiotics as cosmetic ingredients raises many questions. From a formulation standpoint, the first challenge is to maintain these microorganisms alive in a cosmetic formulation. Most cosmetics are water-based and pH neutral or slightly acidic, which can be considered as favorable conditions, but they also contain some ingredients that could affect probiotics ‘survival’: surfactants, chelating agents, glycols, preservatives, fragrance. Moreover, the preservation of cosmetic products from microbial contamination and proliferation is a safety and regulatory requirement. Therefore, the challenge is to maintain probiotics alive in cosmetic products while preventing the growth of microorganisms that could adversely affect the health of the consumers. This can be achieved by different means such as the encapsulation of probiotics, or the use of suitable packaging where the living bacteria are kept separately and mixed with the formulation at the time of use.
From a regulatory standpoint, the ICCR report indicates that “There were no unique regulations governing cosmetic products or ingredients intended to work specifically with the skin’s (or mucosal) commensal microbiome. Rather, such products are subject to the applicable rules and regulations governing cosmetics in each respective jurisdiction, including those covering both safety and product representation (i.e., claims). Several jurisdictions pointed out that while no distinct regulations exist specific to these products there are general quality standard requirements such as microbiological limits which apply to all cosmetic products, including those containing live or viable microorganisms” [
204].
The microbiological limits for cosmetic products are given in an International Standardization Organization (ISO) standard [
265]. This document states that although cosmetics are not required to be sterile, microorganisms present in a product should not cause an adverse effect on consumer safety or product quality. Therefore, the manufacturer must respect the Good Manufacturing Practices and take the necessary precautions to limit the introduction of microorganisms from raw materials, processing, and packaging.
In this standard, microorganisms are considered as contaminants that are unintentionally introduced in the cosmetic product and microbiological limits are established to ensure product quality and consumer safety. Therefore, those limits should not apply to probiotics which are well-characterized microorganisms intentionally introduced in cosmetic products to achieve a cosmetic benefit. However, discussions are still ongoing, and some clarification is needed to allow the use of living bacteria as cosmetic ingredients.
The use of live probiotics is one of the major future applications in the dermo-cosmetic industry but not the only one. Each human has his or her own ‘microbial fingerprint’ that is specific to his or her skin and this specific microbiome may influence its homeostasis. The microbiome is the path to an individualized skincare routine.
In this perspective, personalized microbiome-derived cosmetic solutions that would intervene specifically are the future paradigm for safe, effective, and successful skin/scalp care products.
4. Conclusions
Research is at the dawn of a «new generation» cosmetic that will use the skin’s microbiome to provide lasting products with new efficient performance. To bring to light this rising cosmetics category that harnesses the potential of the cutaneous microbiome, it is essential to dissect the dynamic interactions existing between microorganisms and the interplay host/Microbiome. Researchers would also need to understand the regulatory/safety framework to translate these innovations (Figure 2). However, only rigorous and unbiased experimental approaches considering the specificity of the skin-microbiome environment can be applied. This discovery will be made possible by coupling multi-omics technologies, statistical data mining, and representative 3D skin models. These approaches may provide the opportunity to establish microbiome/skin condition causality and, subsequently, cosmetic solutions. The consideration of subtle regulatory environments and country-specificities will also be of high concern.
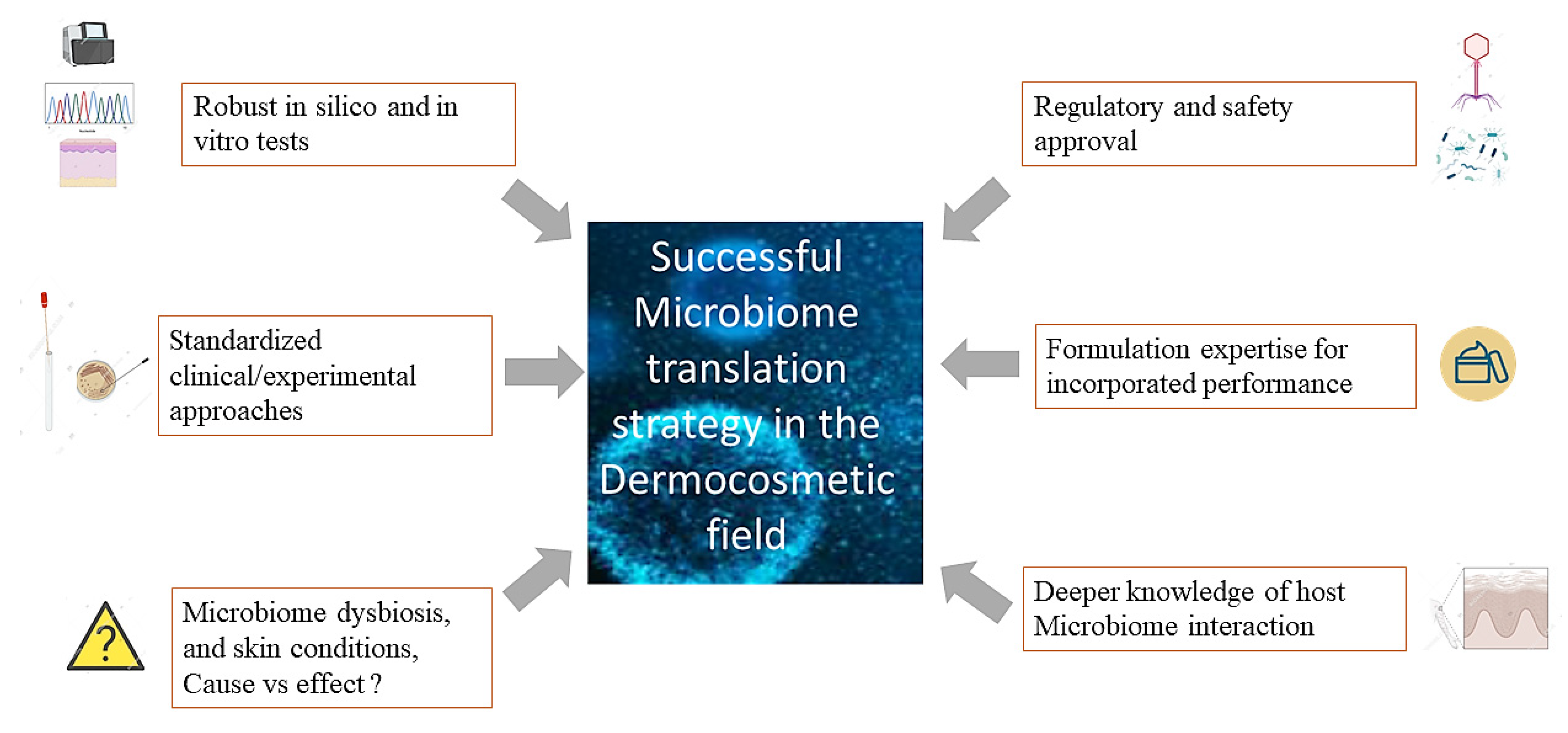
Figure 2. Resuming the best strategy for a successful translation of Microbiome-based concepts into cosmetic products of the future. Combined approaches of multi-omics technologies, powerful data mining tools, and representative 3D vitro skin models associated with standardized and unbiased experimental approaches dedicated to skin Microbiome analysis are key. Harnessing recent scientific breakthroughs and deciphering the famous causality question allied to better characterization of the interaction between the microbiome, the immune system and skin cells in various skin conditions would accelerate the translation. Finally, consideration of regulatory and safety aspects related to these new/targeted Microbiome-derived technologies (postbiotics, phages, probiotics…) and how to leverage their performance in different formulation types is essential.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens11020121

