Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Contact lens materials are typically based on polymer- or silicone-hydrogel, with additional manufacturing technologies employed to produce the final lens. These processes are simply not enough to meet the increasing demands from CLs and the ever-increasing number of contact lens (CL) users. New materials and engineering offer increasing functionality or improved properties over previous generations.
- contact lens
- materials
- biomedical devices
- materials engineering
1. Overview
To manufacture CLs, there must be a suitable polymeric material. This opens an incredible number of possibilities, not only from the range of polymers but to the formula of components within a given recipe. In addition, there can be considerations for the different types of polymerization mechanisms to form the same polymer, such as radical vs. catalytic polymerizations and derivatives. Within this, the polymerization conditions (temperature, initiator type, vessel used, etc.) can be altered to produce the same polymer but with different properties. Finally, the material must be suitable for the manufacturing stages, which include the synthesis, inspection, and packaging processes. The manufacturing stages have their own intricacies, which will be discussed later; thus, it is easy to quickly get lost in the search for the most suitable CL material. The extent of variation in materials is why there is such a wide range of CLs available today, and this has been extensively researched. Figure 1 contains some of the most important factors from a materials science perspective when designing CLs. From this, an assessment of current CL materials’ pros and cons is given in Table 1.
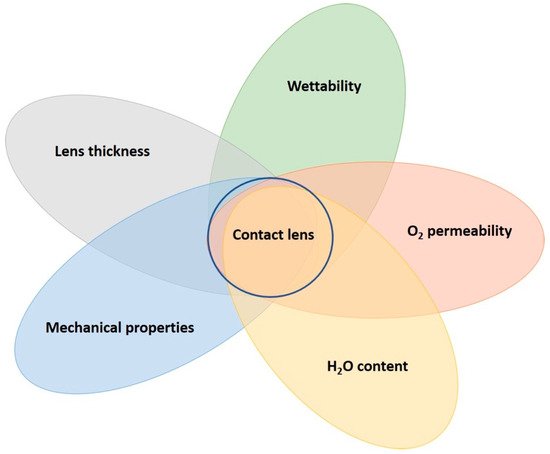
Figure 1. A CL lens is dependent on many parameters from a material science perspective. Stronger emphasis on specific characteristics are required, depending on the specific demand placed on the CL. The final CL material accounts for wear time and comfort. These characteristics are often dependent on the materials, but also includes manufacturing processes, such as plasma treatment.
Table 1. General pros and cons of current CL material classes.
| CL Material | Pros | Cons |
|---|---|---|
| PMMA | Inexpensive, well-understood polymer | No oxygen permeability, inflexible on the eye |
| RGP | High oxygen permeability, durable | Expensive regents, requires hydrophilic comonomer, can be abrasive |
| HEMA hydrogel | Inexpensive, biocompatible, abundant copolymer possibilities | Low oxygen permeability, protein deposition issues |
| Silicone hydrogel | High oxygen permeability, durable, comfortable | Expensive regents, requires hydrophilic comonomer, can be abrasive |
| PVA | Inexpensive, straight-forward manufacturing, biocompatible | Low oxygen permeability, fixed water content |
For clarity, a polymer is a large macromolecule composed of hundreds or thousands of a repeating molecule, called a monomer. Each monomer has a covalent bond between each connecting unit. Some common monomers and polymers used in the production of CLs can be seen in Figure 2. The process by which the polymer is formed is called polymerization. Another reactive molecule, the initiator, is used to begin the polymerization. Polymerization initiators are typically selected based on the interaction with the reactive functional groups within the monomers. Radicals are formed on the breakdown of the initiator, which then induce the polymerization by stripping a radical from the monomer functional group. Now the monomer has a free radical within itself, which reacts with a neighboring monomer functional group by stripping a radical from this group. The original monomer would have then formed a new bond with the new monomer unit, which now has a new radical to continue polymerizing. This propagation step is continuously repeated, thus forming the polymer over time, which extinguishes when there are no more monomers to consume. The polymerization terminates when the radical is quenched in another manner, such as by a radical scavenger. The initiator can be chosen for their practicality, such as ultra-violet (UV) or thermal initiators, which is a huge consideration for the manufacturing method in producing CLs.
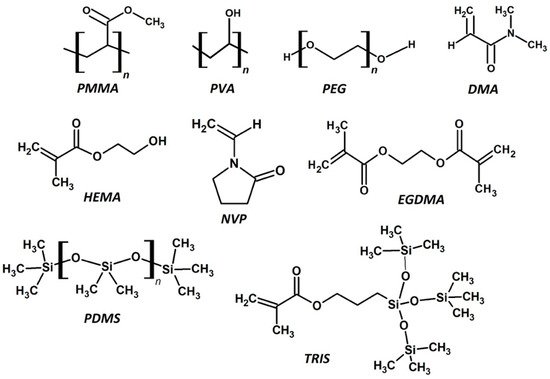
Figure 2. The chemical structures of common monomers and polymers used to produce CLs. This includes some macromonomers and cross-linking agents. PMMA—poly methyl methacrylate, PVA—poly vinyl alcohol, PEG—poly ethylene glycol, DMA—dimethyl methacrylate, HEMA—hydroxy ethyl methacrylate, NVP—N-vinyl pyrrolidone, EGDMA—ethylene glycol dimethacrylate, PDMS—poly dimethyl siloxane, TRIS—3-[tris(trimethylsiloxy)silyl]propyl methacrylate.
2. Polymerization Mechanisms
Free-radical polymerization (FRP) is a type of chain-growth polymerization mechanism. Typically, free-radical polymerizations are often not easily controlled, producing high dispersity (large variations in molecular weights) of the resulting polymers. This can lead to a distribution of the polymer properties. High strain and tensile modulus polymers are often determined by the length of the polymer chains [1]. One advantage of FRP is that it is easy to form gelling networks with, as the polymerization has many initiation sites within the vessel. This allows for the simultaneous growth of many chains which can physically entangle or cross-link to form the gel network. Cross-linking is dependent on one of the monomers containing two functional groups, which enables chemical bonding to two different polymer chains. Cross-linking polymer chains often improves gelling and increases the modulus of the material. To date, most CLs are produced using free-radical polymerization [2][3][4][5][6][7][8]. It is facile and doesn’t require expensive reagents, such as catalysts. Furthermore, unwanted/unreacted chemicals can be removed from the material on post-fabrication cleaning processes. Catalysts are often composed of heavy metals, which is something to avoid for human health.
Today, most CLs are produced from a polymerization of two or more monomers [6][9]. Copolymers incorporate the properties of the individual polymers; consequently, copolymerization is often the first method used in overcoming issues with a single polymer. This principle has governed the development of CLs for many years. For example, silicone polymers are very hydrophobic, despite their high oxygen permeability. Therefore, they are not ideal as a homopolymer CL material. However, copolymers of silicones with a hydrophilic (highly polar) monomer can solve this problem. Copolymerization may also be used to enhance physical properties through the cross-linking of polymer chains by adding molecular weight to the chain. In some cases, adding a soft polymer can reduce the modulus of particularly tough materials (often silicone-based materials). Hydrophobic comonomers add oxygen permeability to materials that require improvement to this property, and silicone-based monomers are often used for this purpose. Table 2 contains the properties of common CL materials and related copolymers, including oxygen permeability, water content, moduli, and wear time. Wear time is from the perspective of the maximum wear time of a CL before eye health issues arise. More exhaustive resources comparing these properties are given elsewhere [10][11]. The effect of copolymerization is particularly noticeable for rigid materials derived from PMMA with a large reduction in the modulus, whereas the hydrogel classes of lenses have a much wider stable range of moduli, but large variations in water content and oxygen permeability.
Table 2. Generalized properties of some common CL materials.
| Material | Oxygen Permeability (Dk/t) | Water Content (wt %) | Modulus (MPa) | Wear Time (days) * |
|---|---|---|---|---|
| PMMA | 0 | 0 | 1000 | <1 |
| PMMA-silicone | 15 | 0 | ||
| Silicone-HEMA (rigid) | 10–100 | 0 | 10 | |
| HEMA hydrogel | 10–50 | 30–80 | 0.2–2 | 1–7 |
| HEMA-NVP | ||||
| HEMA-MMA | ||||
| Silicone (PDMS) hydrogel | 60–200 | 20–55 | 0.2–2 | ~7–28 |
| TRIS-DMA | ||||
| PDMS-HEMA | ||||
| PVA | 10–30 | 60–70 | <1 |
* Maximum wear time without extensive complications to the eye before lens disposal.
Full-density polymers utilize molecular weight and intermolecular forces to provide the strength of the material; this is partly why PMMA has a high modulus. Other factors, such as the lens thickness of the final lens, is important too, whereas the properties of very low-density polymers (10% of full density, 90% air), such as porous materials are sensitive to small changes in cross-linking [12][13]. Another study demonstrated a reduction the Youngs modulus of up to 40% in a 90% porous material due to inefficient cross-linking [14]. This could be relevant to very high-water-content hydrogels on hydration from the final material properties and manufacturing considerations [15]. In fact, Maldonado-Codina and Efron highlighted the need for improving processes between polymerization batches and manufacturing methods. Other factors that are important include homogeneity of the wall vertices, uniform pore size, etc.; all of which are not guaranteed to be consistent with FRP. Moreover, if we consider SCLs to be porous cellular solids, these factors must be considered in future designs [16]. Although FRP has been the workhorse for fabricating excellent CL materials, they may not be producing entirely efficient networks for functional materials [17]. This could be another reason why there are no drug-delivery CLs on the market today, in addition to the many reasons stated by Dixon et al. [18]. Alternative polymerization mechanisms could be of interest to improve the physical properties of CL materials. Other kinds of methods include catalysts or controlled radical polymerization. One controlled method for radical polymerization is chain-transfer polymerization. This has been a popular area of growth in polymer science, including examining the potential in other bio-applications [19][20][21]. The chain-transfer agent accurately mediates the growth of the polymer chain, so that the molecular weight can be pre-designed [1][22][23]. This results in a low-dispersity polymer, meaning the resulting properties and structure are more reliable than FRP (Figure 3). There are many examples of hydrogels produced using chain-transfer agents [24][25][26]. One specific example of reverse addition fragmentation chain transfer (RAFT) polymerization encompasses silicone-based polymers [27]. Other researchers also used RAFT to modify polyacrylic acid pH-responsive hydrogels for drug delivery [26]. This opens the potential of RAFT-synthesized silicone- and conventional hydrogels as CLs. Recently, Zhang et al. synthesized a promising RAFT-polymerized soft contact lens based on polyallyl methacrylate and PEG components [28]. The lenses had low contact angles (<80°), high Dk values (>100 barrers), and elastic moduli ranging from 0.5–1.5 MPa, which are in the range of the CL parameters given in Table 1.
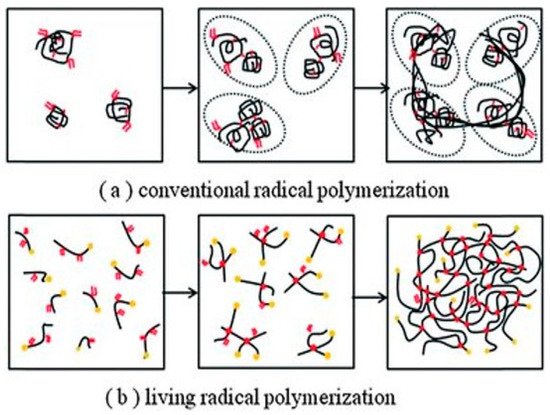
Figure 3. Schematic for the macromolecular structure differences between FRP and RAFT-polymerized materials. The RAFT polymer can be used to create a more ordered structure compared to FRP, which could be important to the macromolecular structure and final application of the material. Republished with the permission of The Royal Society of Chemistry, from Pushing the mechanical strength of PolyHIPEs up to the theoretical limit through living radical polymerization, Y. Luo, A-N. Wang, X. Gao, 8, 2012; permission conveyed through Copyright Clearance Center, Inc. [17].
3. PMMA and Rigid Contact Lenses
3.1. PMMA
Today, PMMA CLs occupy a market share of about 1% [29]; however, they are a useful place to begin to appreciate CLs materials—the properties of polymers that are suitable for ocular wear. One of the biggest issues with PMMA is that it has little to no oxygen permeability. This is due to the lack of mobility of polymer chains preventing the flow of oxygen or internal water to mediate the flow of O2 (Figure 4). This occurs in PMMA due to intermolecular forces, such as dipole–dipole bonding and physical entanglement that is prevalent between polymer chains. The dipoles are created by the negatively charged (electrochemical negative) oxygen compared with the adjacent positively charged (electrochemical positive) carbon and hydrogen atoms. Therefore, neighboring polymer chains can attract each other to provide thermodynamic stability to the polymer. These intermolecular forces also mean that PMMA has low free volume (space between polymer chains), meaning the chains do not rotate or move easily. In addition, PMMA does not contain large pendant chains that prevent the interaction of neighboring chains. All these factors together prevent the flow of oxygen through the polymer. However, functionalization of the PMMA surface can improve the hydrophilicity [30], which would be useful to these CLs.
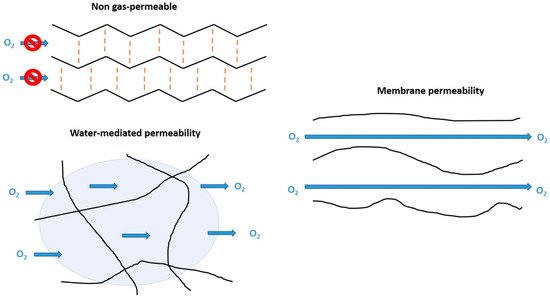
Figure 4. Schematic for gas-permeability mechanisms of CL materials from the perspective of polymer chains. These 2D models represent how oxygen passes (or not) through the molecular structure of the CL material. These schematics do not show factors such as extensive cross-linking or the macromolecular structure that would be present in a 3D structure.
Very low loadings of ZnO quantum dots (0.017 wt %) reduced the transmittance of UV light by 50%, yet retained suitable optical transparency expected of a contact lens (Figure 5). Perhaps the most novel advancement for PMMA lenses was the possibility of developing nanophotonic lenses [31][32]. Acid- and hydroxyl-functionalized fullerenes (C60) were attached to PMMA to try to harness the photo- and electro-activity of the fullerene. However, these nanophotonic lenses have now been a focus of SCL materials instead [33].
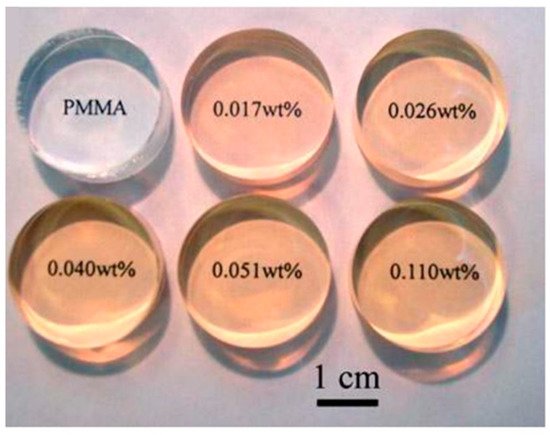
Figure 5. PMMA buttons loaded with ZnO quantum dots. The fabricated buttons would be suitable for lathe-cutting manufacturing to form a final CL. Reprinted (and adapted) with permission from John-Wiley and Sons [34].
3.2. Other Rigid CLs
Modern rigid gas-permeable (RGP) lenses have moved away from PMMA, or significantly reduced the molar fraction of PMMA in many copolymers. Efron wrote an obituary for RGP lenses stating the reasons why these lenses are obsolete [37]. Nevertheless, RGP lenses (including PMMA) still account for about 14% of all lenses fit in the US as of 2017 [29]. Modern research concerning RGP lenses is in regard to their application, treatment, and effect on eye conditions, rather than innovations on the material [38][39][40]. Eggnik et al. reported that a large-diameter RGP lens of unknown composition was used to improve post-surgery treatment for laser in situ keratomileusis (LASIK) patients [41]. The success of RGP lenses lies in their rigidity, causing a reshaping of the cornea, which was useful for some post-surgery treatment [42]. Soft lenses are sometimes unsuitable for treatment as they are very malleable, and therefore will shape to the user’s eye. These other works often use commercially manufactured lenses to carry out their research; therefore, it is more difficult to know the exact composition and methods by which these materials were synthesized. However, it is known that the typical composition of these lenses are based on fluoro silicone or siloxane (such as PDMS and TRIS) acrylate moieties, alongside hydrophilic monomers such as HEMA, NVP, and methacrylic acid (MAA) [11][38]. Baucsh & Lomb were assigned a patent for polysiloxane, copolymerized with urea moieties prepolymers to from RGP lenses [43]. The urea (or carbamides (CO(NH2)2) moieties added hydrophilic properties. These vinyl end-capped prepolymers were then copolymerized with well-known monomers such as NVP, MAA, MMA, and TRIS to form the RGP lens.
4. HEMA-Derived Hydrogels
HEMA and related hydrogels are a high-water content, oxygen-permeable polymeric material. These hydrogels can have water content between 20–80% depending on the comonomers, with a hydrogel composed of only HEMA containing about 38% water. HEMA’s highly polar properties mean these CLs have generally suitable wetting properties, meaning they are comfortable. The oxygen permeability of these gels is suitable for longer wear, but not to the same extent of silicone-based lenses. HEMA-derived hydrogels form an important part of the market, occupying about 22% [29]. HEMA is commonly copolymerized with monomers such as EGDMA, MAA, and NVP. NVP and MAA increase the water content of hydrogels due the strong hydrophilic character arising from amine, carboxylic acid, hydroxyl groups, etc. Therefore, these comonomers also influence the wettability of the surface [44][45]. The mechanical properties can be improved by using a cross-linking molecule, such as ethylene glycol dimethacrylate (EGDMA). EGDMA has two functional groups allowing the formation of covalent bonds between two individual polymer chains. This increases the mass of the polymer dramatically, and improves its ability to form a gel network. However, the crosslinks also reduce the polymer-chain motion, which can be a factor in swelling and oxygen transport [45]. The water content and cross-linking affect the modulus and oxygen permeability of the hydrogels (Figure 6). As such, a balance must be reached between these parameters when designing a CL for a particular application.
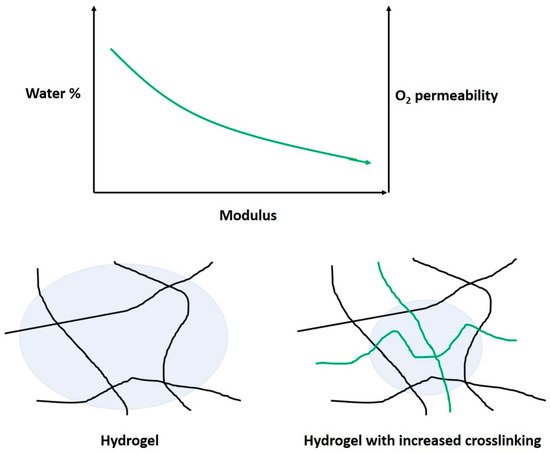
Figure 6. Schematic on the effect of cross-linking on the modulus, water-content percentage, and oxygen permeability. The increased cross-linking prevents (green) the polymer chains from swelling, compared with lower-modulus gels.
Currently, modification of the HEMA-hydrogel structure is paving the way for new lenses, innovations, and applications. Protein deposition on the eye remains a concern, particularly for SCL materials based on HEMA [46][47]. Common comonomers, such as MAA and NVP, increase the protein deposition. Both comonomers introduce hydrophilicity and electrochemical charge to the hydrogel, which attracts the proteins from the tear film [46]. Lord et al. also showed that the protein uptake could affect the water content of a HEMA-MAA hydrogel. This could be related to observations by Tranoudis et al. where a HEMA-MAA hydrogel lost 15% water content across a 6 h period [48]. An interesting report by Borazjani et al. regarding the bacteria adhesion of Pseudomonas aeruginosa, a risk factor in keratitis, to a HEMA-hydrogel was no less than that of a silicone hydrogel [49]. Borazjani et al. also reported that adhesion of the bacteria was more related to the strain of bacteria than the lens material. Another study by Szczotka-Flynn et al. demonstrated that biofilms of bacteria (particularly Pseudomonas aeruginosa) were resistant to the antimicrobial action of CL solutions [50]. Dutta et al. looked into the literature of this area by considering the material type, length of wear, and bacterial strains on the cumulation of the bacteria on contact lenses [51]. New strategies to prevent this have been in development since the early 2000s, including further modification of the lens material. A more detailed and extensive article on antimicrobial strategies was published by Xiao et al. [52].
A surfactant consists of a hydrophobic and hydrophilic component, and is primarily used to promote the reduction in surface tension between two immiscible liquids. Bengani et al. employed the novel use of polymerizable surfactants attached to HEMA hydrogels to enhance wettability and lubricating properties [53]. They achieved a 10° reduction in water-contact angle with about 2.4 wt % surfactant, which was covalently bonded to the hydrogel by UV polymerization. The low-surfactant loading meant that the HEMA-hydrogels remained below 45% water content, which indicates the power of such a technique. In this case, the hydrophilic component interacts with the aqueous tear film and the hydrophobic part remains in the hydrogel (Figure 7). There is much modern literature that still reports high rates of contact lens discontinuation due to discomfort and dryness [54][55][56]. This emphasizes the importance of new techniques, such as surfactant loading/grafting employed by Bengani et al. Other uses of surfactants include use as a drug delivery system for cyclosporine A in modified HEMA-hydrogels [57]. In this case, the surfactant leached out of the hydrogel with the drug encapsulated within surfactant aggregates (Figure 8). This is now becoming a more researched topic, using a larger number of surfactants to deliver drugs [58][59][60][61]. These works have used a diverse range of surfactants, from cationic to non-ionic, with molecular weights ranging from about 400–12,500 g mol−1. This wide variation in surfactant properties is interesting, suggesting that the hydrogel structure is highly adaptable to such modifications.
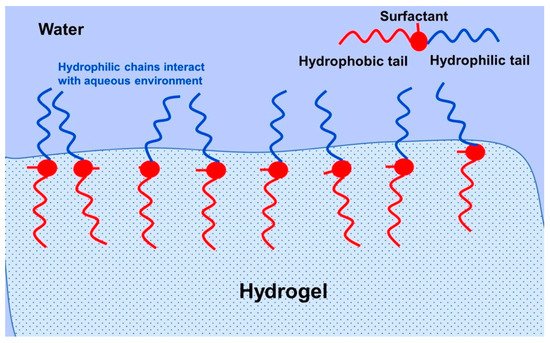
Figure 7. Schematic for the interaction between the aqueous tear film and a surfactant bonded with a hydrogel lens. Reprinted from Journal of Colloid and Interface Science, 445, Bengani, L.C.; Scheiffele, G.W.; Chauhan, A.; Incorporation of polymerizable surfactants in hydroxyethyl methacrylate lenses for improving wettability and lubricity, 60, Copyright 2014, with permission from Elsevier [53].
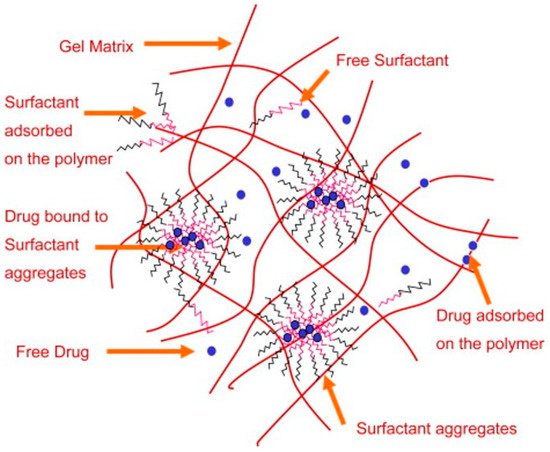
Figure 8. Drug-loaded hydrogel loaded with surfactants. The aggregation and leaching of the surfactant acts as a drug delivery vehicle. Reprinted from Biomaterials, 30, Kapoor, Y.; Thomas, J.C.; Tan, G.; John, V.T.; Chauhan, A. Surfactant-laden soft contact lenses for extended delivery of ophthalmic drugs, 867, Copyright 2008, with permission from Elsevier [57].
In summary, HEMA-hydrogel CLs are in an exciting place. The ability to design new materials is due to the flexible hydrogel structure composed from many functional groups. Essentially, the core of the hydrogel has remained relatively unchanged, due to the principal properties that matter to CLs, such as oxygen permeability, water content, wettability, etc. Modern HEMA hydrogel lenses have evolved through modification of the hydrogel structure through techniques such as encapsulation and grafting.
5. Silicone-Derived Hydrogels
Silicone-based hydrogels are the most common type of CL material today, occupying about 64% of the US market [29]. This includes silicone, silioxanes, fluorosiloxanes, and derivative materials. Their popularity is linked to the fact that these CLs have the highest oxygen permeability of all contact lens materials (>100 Dk, typically). Silicone CLs are often durable, originating from the high Si–O bonding energy, often with a higher modulus than conventional polymer hydrogels. This typically applies to RGP lenses, as the modern silicone hydrogel has a similar modulus to HEMA-derived hydrogels. The high modulus is linked to causing irritation to the eye, such as the conjunctiva of the inner eyelid [11]. In fact, the discomfort and dryness of silicone-based lenses are two of the main reasons for users’ discontinuation [54][56][62][63]. Specifically, the wettability of the lens and the incompatibility with the cornea environment in vivo requires detailed study to improve these parameters [64]. The patent literature contains much about improving the wettability of lenses, with companies such as CooperVision [65] and Johnson and Johnson [66] having filed for patents to develop such lenses. This includes using many combinations of comonomers with silicone monomers. However, similarly to the preceding sections, modern silicone lenses have not deviated from the base materials since they were first developed. Nowadays, new chemical techniques look to evolve the silicone CL material beyond its predecessors.
Chitosan is a naturally derived polymer (from chitin) with high bioavailability originating from the hydroxyl and amine groups within the structure, lending itself to lens modification. They modified the plasma-treated silicone surface to create negatively charged groups, which allowed the formation of the positively charged chitosan layer. Then, the negatively charged hyaluronic acid formed a layer on this surface to form alternative layers. The contact angle was reduced from 90° in the mother lens to up to 50° in the PEM-modified lenses. Similarly, a modified chitosan was also used successfully by Tian et al. to form a chitosan layer on the surface of the lens (Figure 9) [67]. Hydroxypropyl trimethyl ammonium chloride chitosan is a quaternary ammonium salt and is positively charged, which facilitates the formation of self-assembled layers. These pendant chemical groups could lead to further modification of the lens. Thissen et al. modified silicone lenses with a poly ethylene oxide (PEO) graft to the surface for anti-biofouling properties [68].
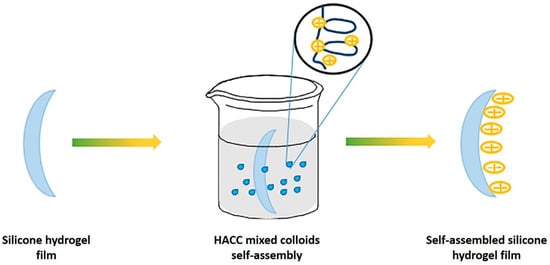
Figure 9. Schematic for the adhesion of a self-assembled layer onto the surface of silicone hydrogel. Reprinted (and adapted) from Colloids and Surfaces A, 558, Tian, L.; Wang, X.; Qi, J.; Yao, Q.; Oderinde, O.; Yao, C.; Song, W.; Shu, W.; Chen, P.; Wang, Y. Improvement of the surface wettability of silicone hydrogel films by self-assembled hydroxypropyltrimethyl ammonium chloride chitosan mixed colloids, 422, Copyright 2018, with permission from Elsevier [67].
In summary, silicone hydrogel lenses are targets for highly biocompatible coatings that can be grafted/encapsulated to the surface of, or within, the lenses by chemistry techniques. In these experiments, the performance of the lens was improved, particularly towards anti-biofouling applications. Hydrophilic anti-biofouling materials are being applied more often to silicone hydrogel CLs [69]. This emphasizes the innovations which silicone-based hydrogel lens materials are undergoing. For silicone hydrogel, there is a two-fold benefit of these anti-biofouling materials: (1) These hydrophilic grafts/modifications are the route to anti-biofouling behavior, and; (2) they are hydrophilic, increasing the wetting character of the lens. This effect is also relevant to HEMA-based lenses; however, it is more impactful to silicone lenses, given their inherent wettability disadvantages and market size. Continued improvements to these materials will result in continued market dominance for silicone-based hydrogel CLs.
6. Other Contact Lens Materials
6.1. Polyvinyl Alcohol Hydrogels
PVA is a synthetic polymer that contains many hydroxy (–OH) groups, one in each repeating monomer unit. This is the source of PVA’s excellent hydrophilic and biocompatible properties [70]. As such, it is clear why this material is an interesting contact lens material. PVA hydrogels were of attention to several researchers in the early 1990s [71][72]. Even without modification, PVA CL hydrogels were also shown to have lower protein absorption rates than HEMA and MMA/VP hydrogels [72]. However, it wasn’t until the late 1990s that this material broke into the market in the form of the Nelfilcon A lens [73]. These lenses have a Dk of about 26 barrers and high wettability, which is acceptable for daily wear. Produced by CIBA vision, the PVA hydrogel was synthesized using water as the solvent and produced inside a transparent mold to allow UV initiation of the polymer solution. Water was an environmentally friendly choice of solvent which also did not hinder the polymerization stages. Buhler et al. formed the PVA hydrogel by adding a new functional group to facilitate cross-linking between chains [73]. In acidic conditions and a suitably reactive dialdehyde group, PVA can be functionalized with a stable cyclic-acetal group. This can then crosslink with other PVA chains to form a crosslinked hydrogel. More recently, PVA has been used as a tool for producing more comfortable lenses [74][75] or to facilitate the loading of a colored pigment into the lens [76]. One of the comfort mechanisms include elution of an unbound PVA polymer from the contact lens into the tear film. Non-crosslinked PVA with an approximately 47,000 molecular weight was soaked into a cross-linked PVA lens. The high molecular weight and bioavailability meant that PVA leeched out of the lens to lubricate the eye as a sort of artificial tear component [75][77]. Researchers identified that the higher molecular weight species took longer to elute from the lens than lower molecular weight species. Although these examples are strictly not using PVA hydrogels, they highlight the importance of this material for other ophthalmic applications. This even extends to areas such as corneal replacement [78].
There has been some investigation into the modification of PVA hydrogel CLs. Xu et al. modified the PVA structure with β-cyclodextrins (β-CDs) to improve drug-loading of puerarin and acetazolamide [79]. They functionalized both the β-CDs and PVA with a methacrylate moiety with N,N-dimethyl-4-pyridinamine and glycidyl methacrylate to achieve the necessary functionality. Then, both methacrylated species were copolymerized to form the hydrogel. This method also resulted in incorporation of 30 wt % of β-cyclodextrin. Sun et al. also used a functionalized β-CD polymer incorporated into a PVA film for the delivery of voriconazole [80]. A β-CD solution containing dissolved voriconazole was mixed with a PVA solution and then used to form electrospun nanofibers. PVA cross-linked with cellulose offers another a potential route to improved contact lens materials [81][82]. Cellulose is a naturally occurring polymer with enormous potential for bio-applications [83]. Mihranyan chemically bound the cellulose microcrystal “whiskers” onto the surface of PVA using a 2,2,6,6-tetramethyl-1-piperidine oxoammonium salt (TEMPO)-mediated surface oxidation [82]. The TEMPO technique is a special technique used for regioselective oxidation of cellulose to preserve crystallinity [84]. The technique only functionalizes the surface of the material. Although the physical properties of the PVA hydrogels improved, the opaque material would not be ideal as a CL material. Tummala et al. incorporated nanocellulose, a transparent version of cellulose, as nanofibrils or nanocrystals into a PVA hydrogel, improving the physical properties [81]. In addition, the lens retained excellent optical properties, with >90% transparency to visible light (Figure 10). They used a mixed solvent system of water:dimethyl sulfoxide (DMSO) at ratios between 60–80% DMSO content to facilitate the solvation of the nanocellulose components. These nanocellulose PVA hydrogels were investigated for their light-scattering properties by Tummala et al. in another work [85]. The hydrogel composition and angle of light affected the scattering of light in the range of 3% to 40%, with the nanocellulose-functionalized PVA hydrogel reducing the scattering. The nanocellulose hydrogels were then used to encapsulate acrylic-acid-functionalized chitosan nanoparticles as a vehicle for a lysozyme-triggered release of a model drug. Lysozyme (0.2 mM)-mediated hydrolysis of the chitosan-functionalized nanoparticle would trigger the release of the drug.
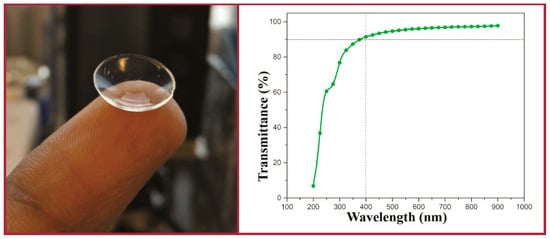
Figure 10. Optical image (left) and the transmittance data (right) for PVA hydrogels incorporating nanocellulose. Reprinted (adapted) with permission from Tummala, G.K.; Rojas, R.; Mihranyan, A.; Poly(vinyl alcohol) hydrogels reinforced with nanocellulose for ophthalmic applications: general characteristics and optical properties, Journal of Physical Chemistry B, 2016, 120, 13094. Copyright 2016 American Chemical Society [81].
The PVA hydrogel is a relatively new class of hydrogel lens compared with silicone- and HEMA-hydrogels. The low cost, high bioavailability, and wettability properties mean PVA hydrogels will remain important to the CL industry. This means there is a lot of design space for investigation into modifying the structure to improve lens functionality. There are an increasing number of modification techniques and materials that have yet to be explored with PVA hydrogels. The secondary alcohol groups within the polymer chain are very easily modified for grafting, as shown by cellulose or β-cyclodextrin incorporation. As with HEMA- and silicone-hydrogels, the grafting or encapsulation of anti-microbial agents is just one of many interesting avenues of research that is possible for PVA hydrogels.
6.2. Hyaluronic-Acid-Modified Hydrogels
Hyaluronic acid (HA) is a hydroscopic biopolymer that naturally occurs within the human body. HA has already been mentioned in previous sections. Here, we explain why this material is significantly impacting CL research. This is an important material for a wide range of tissue-engineering purposes [86]. From the chemical structure, we can see why it is useful for incorporating into CL materials (Figure 11). The amino acid and hydroxyl groups present in each repeating unit provide the necessary hydrophilic character, leading to high biocompatibility. As such, this material has been of interest in developing ophthalmic treatments, such as lubricating solution [87] or contact lens modification [88]. The incorporation of HA was shown not to affect the surface morphology of the CL even after 12 h of wear, showing the stability of these modifications [89]. HA is typically a graft/encapsulating material to other established CL hydrogels. The commercial success of HA is apparent with the Bausch&Lomb Biotrue™ solution and Open30 (by Safilens) lenses incorporating HA as a lubricating agent. Other ophthalmic treatments and modifications include HA use as a treatment for dry eyes, [90][91] modification of the lens material [92][93][94], and drug-delivery mechanism [95][96][97].
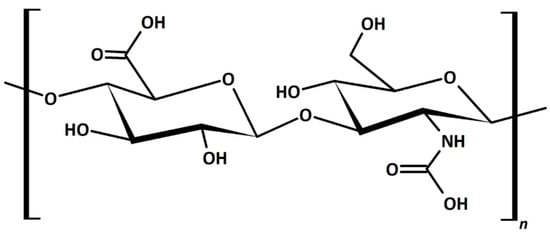
Figure 11. Chemical structure of hyaluronic acid. The repeating unit within the polymer is hydrophilic with many highly polar groups, such as the amine, acid, and hydroxy groups.
HA was investigated as a reusable wetting agent, and considered as a vehicle for the delivery of timolol within a silicone hydrogel lens [98]. HA is hydroscopic, meaning it was used to add water content to a material without a significant reduction in the other hydrogel components of DMA and TRIS [94]. All of the silicone hydrogels Paterson et al. produced had a water contact angle of less than 50°, emphasizing the wettability qualities of HA. The wetting agent was a copolymer of PEG and HA, which was first copolymerized before being soaked into the HA-modified silicone hydrogels. Van Beek et al. crosslinked/physically entrapped HA into HEMA hydrogels by using 1-ethyl-3-(3-dimethylaminopropyl)-carbondiimide (EDC)/diaminobutane-4 dendimer to provide the necessary functionality for a reaction [93]. Furthermore, HA in very low amounts (5 g/L solution) reduced the water contact angle of the hydrogels by about 15–25°. These HA-modified hydrogels also showed less lysozyme absorption, which can often be a criticism of HEMA-derived hydrogels. However, large molecular weight (Mw) HA (169 kDa) had reduced optical transparency between 500–800 nm versus the lower Mw HA (35 kDa). On the other hand, the 169 kDa HA was responsible for the lowest amount of protein deposition. Korogiannaki et al. covalently bonded HA to the surface of HEMA by using a nucleophilic Michael-addition thiol-ene “click” chemical reaction [99]. These lenses retained an optical transparency level of >92%, and decreased the water contact angle and rate of lens dehydration. A more detailed mechanism of the covalent bonding of HA to the HEMA hydrogel structure was presented by Deng et al. [100] “Click” chemistry was used with adipic acid dihydrazde (ADH) to anchor HA to HEMA. HEMA was first oxidized to provide the functionality for bonding the ADH-HA anchor. The lens properties virtually remained unchanged, such as the modulus and optical properties (Figure 12). They also measured a reduction in the rate of dehydration of the lens compared with the unmodified lenses. Weeks et al. used UV light to covalently bond a methacrylate modified HA to HEMA and silicone hydrogel lenses [101]. In all cases, the HA-modified hydrogels had a greatly reduced water-contact angle and reduction of lysozyme absorption. They also pointed out that HA with little or no methacrylation on the surface would not prevent the interaction of the protein with the surface of the hydrogel, whereas if HA was entrapped inside the hydrogel, the protein could not interact with the surface at as many points, preventing its deposition.
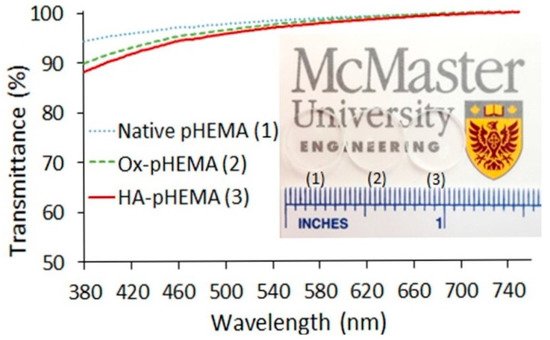
Figure 12. Optical properties of HA-HEMA, oxidized-HEMA (OX-HEMA), and unmodified HEMA lenses. The transparent hydrogels can be seen just above the ruler markings. Reprinted (adapted) with permission from Deng, X.; Korogiannaki, M.; Rastegari, B.; Zhang, J.; Chen, M.; Fu, Q.; Sheardown, H.; Filipe, C.D.M.; Hoare, T. “Click” chemistry-tethered hyaluronic acid-based contact lens coatings improve lens wettability and lower protein adsorption, ACS Applied Materials and Interfaces, 2016, 8, 22064. Copyright 2016 American Chemical Society [100].
HA is an increasingly popular material for the modification of both HEMA-hydrogel and silicone-hydrogel CLs. The high bioavailability and straightforward incorporation methods have already seen recent commercial success for HA. In these cases, HA was a lubricating agent rather than any physical/chemical modification of the lens material. Recent works regarding HA are exciting, demonstrating the ability that HA can alter, tune, or improve the properties of contact lenses. HA can be entrapped or chemical-bound to the hydrogel to provide increased wettability or resistance to protein deposition. One of the most limiting factors for the wider application of HA is the high cost. Although dilution can reduce the cost, the effectiveness of HA will diminish. Once this issue has been overcome, HA will be commonly incorporated into new lenses.
This entry is adapted from the peer-reviewed paper 10.3390/ma12020261
References
- Perkins, W.G.; Capiati, N.J.; Porter, R.S. The effect of molecular weight on the physical and mechanical properties of ultra-drawn high density polyethylene. Polym. Eng. Sci. 1976, 16, 200–203.
- Mitchell, D.D. Wettable Silicone Resin Optical Devices and Curable Compositions Therefor. U.S. Patent 4487905, 11 December 1984.
- Gaylord, N.G. Oxygen-Permeable Contact Lens Composition, Methods and Article of Manufacture. U.S. Patent 3808178, 30 April 1974.
- Rosa dos Santos, J.F.; Alvarez-Lorenzo, C.; Silva, M.; Balsa, L.; Couceiro, J.; Torres-Labandeira, J.J.; Concheiro, A. Soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery. Biomaterials 2009, 30, 1348–1355.
- Seinder, L.; Spinelli, H.J.; Ali, M.I.; Weintraub, L. Silicone-Containing Contact Lens Polymers, Oxygen Permeable Contact Lenses and Methods for Making There Lenses and Treating Patients with Visual Impairment. U.S. Patent 5331067, 19 July 1994.
- Hahn, D.; Johansson, G.A.; Ruscio, D.V.; Blank, C.E. Method for Manufacturing Hydrophilic Contact Lenses. U.S. Patent 4983332, 1 January 1991.
- Tanaka, K.; Takahashi, K.; Kanada, M.; Kanome, S.; Nakajima, T. Copolymer for Soft Contact Lens, Its Preparation and Soft Contact Lens Made Thereof. U.S. Patent 4139513, 13 February 1979.
- Keeley, E.M. Method of Manufacturing Soft Contact Lens Buttons. U.S. Patent 4931228, 5 June 1990.
- Lin, C.H.; Yeh, Y.H.; Lin, W.C.; Yang, M.C. Novel silicone hydrogel based on PDMS and PEGMA for contact lens application. Colloids Surf. B Biointerfaces 2014, 123, 986–994.
- Philips, A.J.; Speedwell, L.; Morris, J. (Eds.) Contact Lenses, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2007.
- Efron, N. (Ed.) Contact Lens Practice, 3rd ed.; Elsevier: Edinburgh, UK, 2018.
- Zhao, J.; Mayumi, K.; Creton, C.; Narita, T. Rheological properties of tough hydrogels based on an associating polymer with permanent and transient crosslinks: Effects of crosslinking density. J. Rheol. (N. Y.) 2017, 61, 1371–1383.
- Narita, T.; Mayumi, K.; Ducouret, G.; Hébraud, P. Viscoelastic properties of poly(vinyl alcohol) hydrogels having permanent and transient cross-links studied by microrheology, classical rheometry, and dynamic light scattering. Macromolecules 2013, 46, 4174–4183.
- Musgrave, C.S.A.; Nazarov, W.; Bazin, N. The effect of para-divinyl benzene on styrenic emulsion-templated porous polymers: A chemical Trojan horse. J. Mater. Sci. 2017, 52, 3179–3187.
- Maldonado-Codina, C.; Efron, N. Impact of manufacturing technology and material composition on the mechanical properties of hydrogel contact lenses. Ophthalmic Physiol. Opt. 2004, 24, 551–561.
- Ashby, M.F. The properties of foams and lattices. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 15–30.
- Luo, Y.; Wang, A.-N.; Gao, X. Pushing the mechanical strength of PolyHIPEs up to the theoretical limit through living radical polymerization. Soft Matter 2012, 8, 1824–1830.
- Dixon, P.; Shafor, C.; Gause, S.; Hsu, K.-H.; Powell, K.C.; Chauhan, A. Therapeutic contact lenses: A patent review. Expert Opin. Ther. Pat. 2015, 25, 1117–1129.
- Fairbanks, B.D.; Gunatillake, P.A.; Meagher, L. Biomedical applications of polymers derived by reversible addition—Fragmentation chain-transfer (RAFT). Adv. Drug Deliv. Rev. 2015, 91, 141–152.
- Pai, T.S.C.; Barner-Kowollik, C.; Davis, T.P.; Stenzel, M.H. Synthesis of amphiphilic block copolymers based on poly(dimethylsiloxane) via fragmentation chain transfer (RAFT) polymerization. Polymer (Guildf.) 2004, 45, 4383–4389.
- Abdollahi, E.; Khalafi-Nezhad, A.; Mohammadi, A.; Abdouss, M.; Salami-Kalajahi, M. Synthesis of new molecularly imprinted polymer via reversible addition fragmentation transfer polymerization as a drug delivery system. Polymer (U. K.) 2018, 143, 245–257.
- Junkers, T.; Lovestead, T.M.; Barner-Kowollik, C. The RAFT process as a kinetic tool: Accessing fundamental parameters of free radical polymerization. In Handbook of RAFT Polymerization; Barner-Kowollik, C., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 105–144.
- Krstina, J.; Moad, G.; Rizzardo, E.; Winzor, C.L.; Berge, C.T.; Fryd, M. Narrow Polydispersity Block Copolymers by Free-Radical Polymerization in the Presence of Macromonomers. Macromolecules 1995, 28, 5381–5385.
- Bivigou-Koumba, A.M.; Görnitz, E.; Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. Thermoresponsive amphiphilic symmetrical triblock copolymers with a hydrophilic middle block made of poly(N-isopropylacrylamide): Synthesis, self-organization, and hydrogel formation. Colloid Polym. Sci. 2010, 288, 499–517.
- Hemp, S.T.; Smith, A.E.; Bunyard, W.C.; Rubinstein, M.H.; Long, T.E. RAFT polymerization of temperature- and salt-responsive block copolymers as reversible hydrogels. Polymer (Guildf.) 2014, 55, 2325–2331.
- Liu, J.; Cui, L.; Kong, N.; Barrow, C.J.; Yang, W. RAFT controlled synthesis of graphene/polymer hydrogel with enhanced mechanical property for pH-controlled drug release. Eur. Polym. J. 2014, 50, 9–17.
- Guan, C.M.; Luo, Z.H.; Qiu, J.J.; Tang, P.P. Novel fluorosilicone triblock copolymers prepared by two-step RAFT polymerization: Synthesis, characterization, and surface properties. Eur. Polym. J. 2010, 46, 1582–1593.
- Zhang, C.; Liu, Z.; Wang, H.; Feng, X.; He, C. Novel Anti-Biofouling Soft Contact Lens: L -Cysteine Conjugated Amphiphilic Conetworks via RAFT and Thiol—Ene Click Chemistry. Macromol. Biosci. 2017, 17, 1600444.
- Nichols, J. Contact Lenses 2017. In Contact Lens Spectrum; PentaVision LLC: Ambler, PA, USA, 2018; pp. 20–25.
- Ozgen, O.; Hasirci, N. Modification of Poly(methyl methacrylate) Surfaces with Oxygen, Nitrogen and Argon Plasma. J. Biomater. Tissue Eng. 2014, 4, 479–487.
- Koruga, D.; Stamenković, D.; Djuricic, I.; Mileusnic, I.; Šakota, J.; Bojović, B.; Golubovoć, Z. Nanophotonic Rigid Contact Lenses: Engineering and Characterization. Adv. Mater. Res. 2013, 633, 239–252.
- Tomić, M.; Munćan, J.; Stamenković, D.; Jokanović, M.; Matija, L. Biocompatibility and cytotoxicity study of nanophotonic rigid gas permeable contact lens material. J. Phys. Conf. Ser. 2013, 429.
- Mitrović, A.D.; Miljković, V.M.; Popović, D.P.; Koruga, D.L. Mechanical properties of nanophotonic soft contact lenses based on poly (2-hydrohzethil methacrylate) and fullerenes. Struct. Integr. Life 2016, 16, 3–6.
- Li, S.; Toprak, M.S.; Jo, Y.S.; Dobson, J.; Kim, D.K.; Muhammed, M. Bulk Synthesis of Transparent and Homogeneous Polymeric Hybrid Materials with ZnO Quantum Dots and PMMA. Adv. Mater. 2007, 19, 4347–4352.
- Van der Worp, E.; Bornman, D.; Ferreira, D.L.; Faria-Ribeiro, M.; Garcia-Porta, N.; González-Meijome, J.M. Modern scleral contact lenses: A review. Contact Lens Anterior Eye 2014, 37, 240–250.
- Ko, J.; Cho, K.; Han, S.W.; Sung, H.K.; Baek, S.W.; Koh, W.-G.; Yoon, J.S. Hydrophilic surface modification of poly(methyl methacrylate)-based ocular prostheses using poly(ethylene glycol) grafting. Colloids Surf. B Biointerfaces 2017, 158, 287–294.
- Efron, N. Obituary—Rigid contact lenses. Contact Lens Anterior Eye 2010, 33, 245–252.
- Haluk, E.; Nazan, E. Corneal endothelial polymegethism and pleomorphism induced by daily-wear rigid gas-permeably contact lenses. CLAO J. 2002, 28, 40–43.
- Shokrollahzadeh, F.; Hashemi, H.; Jafarzadehpur, E.; Mirzajani, A.; Khabazkhoob, M.; Yekta, A.; Asgari, S. Corneal aberration changes after rigid gas permeable contact lens wear in keratokonic patients. J. Curr. Ophthalmol. 2016, 28, 194–198.
- Yuksel Elgin, C.; Iskeleli, G.; Aydin, O. Effects of the rigid gas permeable contact lense use on tear and ocular surface among keratoconus patients. Contact Lens Anterior Eye 2018, 41, 273–276.
- Eggink, F.A.G.J.; Beekhuis, W.H.; Nuijts, R.M.M.A. Rigid gas-permeable contact lens fitting in LASIK patients for the correction of multifocal corneas. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 361–366.
- Vincent, S.J.; Alonso-Caneiro, D.; Collins, M.J. Corneal changes following short-term miniscleral contact lens wear. Contact Lens Anterior Eye 2014, 37, 461–468.
- Lai, Y.-C.; Bonafini, J.A., Jr. Rigid Gas Permeable Lens Material. U.S. Patent 7344731B2, 18 March 2008.
- Maldonado-Codina, C.; Efron, N. Dynamic wettability of pHEMA-based hydrogel contact lenses. Ophthalmic Physiol. Opt. 2006, 26, 408–418.
- Seo, E.; Kumar, S.; Lee, J.; Jang, J.; Park, J.H.; Chang, M.C.; Kwon, I.; Lee, J.S.; Huh, Y. il Modified hydrogels based on poly(2-hydroxyethyl methacrylate) (pHEMA) with higher surface wettability and mechanical properties. Macromol. Res. 2017, 25, 704–711.
- Lord, M.S.; Stenzel, M.H.; Simmons, A.; Milthorpe, B.K. The effect of charged groups on protein interactions with poly(HEMA) hydrogels. Biomaterials 2006, 27, 567–575.
- Luensmann, D.; Jones, L. Protein deposition on contact lenses: The past, the present, and the future. Contact Lens Anterior Eye 2012, 35, 53–64.
- Tranoudis, I.; Efron, N. Parameter stability of soft contact lenses made from different materials. Contact Lens Anterior Eye 2004, 27, 115–131.
- Borazjani, R.N.; Levy, B.; Ahearn, D.G. Relative primary adhesion of Pseudomonas aeruginosa, Serratia marcescens and Staphylococcus aureus to HEMA-type contact lenses and an extended wear silicone hydrogel contact lens of high oxygen permeability. Contact Lens Anterior Eye 2004, 27, 3–8.
- Szczotka-flynn, L.B.; Imamura, Y.; Chandra, J.; Yu, C.; Mukherjee, P.K.; Pearlman, E.; Ghannoum, M.A. Increased resistance of contact lens related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea 2009, 28, 918–926.
- Dutta, D.; Cole, N.; Willcox, M. Factors influencing bacterial adhesion to contact lenses. Mol. Vis. 2012, 18, 14–21.
- Xiao, A.; Dhand, C.; Leung, C.M.; Beuerman, R.W.; Ramakrishna, S.; Lakshminarayanan, R. Strategies to design antimicrobial contact lenses and contact lens cases. J. Mater. Chem. B 2018, 6, 2171–2186.
- Bengani, L.C.; Scheiffele, G.W.; Chauhan, A. Incorporation of polymerizable surfactants in hydroxyethyl methacrylate lenses for improving wettability and lubricity. J. Colloid Interface Sci. 2015, 445, 60–68.
- Chalmers, R. Overview of factors that affect comfort with modern soft contact lenses. Contact Lens Anterior Eye 2014, 37, 65–76.
- Sulley, A.; Young, G.; Hunt, C. Factors in the success of new contact lens wearers. Contact Lens Anterior Eye 2017, 40, 15–24.
- Dumbleton, K.; Woods, C.A.; Jones, L.; Fonn, D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens Sci. Clin. Pract. 2013, 39, 93–99.
- Kapoor, Y.; Thomas, J.C.; Tan, G.; John, V.T.; Chauhan, A. Surfactant-laden soft contact lenses for extended delivery of ophthalmic drugs. Biomaterials 2009, 30, 867–878.
- Maulvi, F.A.; Desai, A.R.; Choksi, H.H.; Patil, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Effect of surfactant chain length on drug release kinetics from microemulsion-laden contact lenses. Int. J. Pharm. 2017, 524, 193–204.
- Bengani, L.C.; Chauhan, A. Extended delivery of an anionic drug by contact lens loaded with a cationic surfactant. Biomaterials 2013, 34, 2814–2821.
- Kapoor, Y.; Bengani, L.C.; Tan, G.; John, V.; Chauhan, A. Aggregation and transport of Brij surfactants in hydroxyethyl methacrylate hydrogels. J. Colloid Interface Sci. 2013, 407, 390–396.
- Sahoo, R.K.; Biswas, N.; Guha, A.; Sahoo, N.; Kuotsu, K. Nonionic surfactant vesicles in ocular delivery: Innovative approaches and perspectives. Biomed Res. Int. 2014, 2014.
- Wolffsohn, J.; Hall, L.; Mroczkowska, S.; Hunt, O.A.; Bilkhu, P.; Drew, T.; Sheppard, A. The influence of end of day silicone hydrogel daily disposable contact lens fit on ocular comfort, physiology and lens wettability. Contact Lens Anterior Eye 2015, 38, 339–344.
- Stapleton, F.; Stretton, S.; Papas, E.; Skotnitsky, C.; Sweeney, D.F. Silicone hydrogel contact lenses and the ocular surface. Ocul. Surf. 2006, 4, 24–43.
- Keir, N.; Jones, L. Wettability and Silicone hydrogel lenses: A review. Eye Contact Lens Sci. Clin. Pract. 2013, 39, 100–108.
- Chen, C.; Hong, Y.; Manesis, N. Wettable Silicone Hydrogel Contact Lenses and Related Compositions and Methods. U.S. Patent 7572841, 11 August 2009.
- Steffen, R.; Mccabe, K.; Turner, D.; Alli, A.; Young, K.; Schnider, C.; Hill, G.A. Soft Contact Lenses Displaying Superior on-Eye Comfort. U.S. Patent 7461937B2, 9 December 2008.
- Tian, L.; Wang, X.; Qi, J.; Yao, Q.; Oderinde, O.; Yao, C.; Song, W.; Shu, W.; Chen, P.; Wang, Y. Improvement of the surface wettability of silicone hydrogel films by self- assembled hydroxypropyltrimethyl ammonium chloride chitosan mixed colloids. Colloids Surf. A 2018, 558, 422–428.
- Thissen, H.; Gengenbach, T.; du Toit, R.; Sweeney, D.F.; Kingshott, P.; Griesser, H.J.; Meagher, L. Clinical observations of biofouling on PEO coated silicone hydrogel contact lenses. Biomaterials 2010, 31, 5510–5519.
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer (Guildf.) 2010, 51, 5283–5293.
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1451–1457.
- Goldenberg, M. Polyoxirane Crosslinked Polyvinyl alcohol Hydrogel Contact Lens. Patent EP0189375B1, 1 June 1994.
- Kita, M.; Ogura, Y.; Honda, Y.; Hyon, S.-H.; Cha, W.-I.; Ikada, Y. Evaluation of polyvinyl alcohol hydrogel as a soft contact lens material. Graefe’s Arch. Clin. Exp. Ophthalmol. 1990, 228, 533–537.
- Buhler, N.; Haerri, H.P.; Hofmann, M.; Irrgang, C.; Muhlebach, A.; Muller, B.; Stockinger, F. Nelfilcon A, a new material for contact lenses. Chimia (Aarau) 1999, 53, 269–274.
- Winterton, L.C. Contact Lenses with Improved Wearing Comfort. U.S. Patent US8030369B2, 4 October 2011.
- Winterton, L.C.; Lally, J.M.; Sentell, K.B.; Chapoy, L.L. The Elution of Poly (vinyl alcohol) From a Contact Lens: The Realization of a Time Release Moisturizing Agent/Artificial Tear. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80B, 424–432.
- Francis, C.A.; Turek, R.C.; Keeley, D.E. Contact Lens with PVA Cover Layer. U.S. Patent 6890075B2, 10 May 2005.
- Peterson, R.C.; Wolffsohn, J.S.; Nick, J.; Winterton, L.; Lally, J. Clinical performance of daily disposable soft contact lenses using sustained release technology. Contact Lens Anterior Eye 2006, 29, 127–134.
- Hou, Y.; Chen, C.; Liu, K.; Tu, Y.; Zhang, L.; Li, Y. Preparation of PVA hydrogel with high-transparence and investigations of its transparent mechanism. RSC Adv. 2015, 5, 24023–24030.
- Xu, J.; Li, X.; Sun, F.; Cao, P. PVA Hydrogels Containing β-Cyclodextrin for Enhanced Loading and Sustained Release of Ocular Therapeutics. J. Biomater. Sci. Polym. Ed. 2010, 21, 1023–1038.
- Sun, X.; Yu, Z.; Cai, Z.; Yu, L.; Lv, Y. Voriconazole Composited Polyvinyl Alcohol/Hydroxypropyl-β-Cyclodextrin Nanofibers for Ophthalmic Delivery. PLoS ONE 2016, 11, e0167961.
- Tummala, G.K.; Rojas, R.; Mihranyan, A. Poly(vinyl alcohol) Hydrogels Reinforced with Nanocellulose for Ophthalmic Applications: General Characteristics and Optical Properties. J. Phys. Chem. B 2016, 120, 13094–13101.
- Mihranyan, A. Viscoelastic properties of cross-linked polyvinyl alcohol and surface-oxidized cellulose whisker hydrogels. Cellulose 2013, 20, 1369–1376.
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393.
- Chang, P.S.; Robyt, J.F. Oxidation of Primary Alcohol Groups of Naturally Occurring Polysaccharides with 2,2,6,6-Tetramethyl-1-Piperidine Oxoammonium Ion. J. Carbohydr. Chem. 1996, 15, 819–830.
- Ubholz, B.; Chröder, S.V.E.N.S.; Ihranyan, A.L.M. Light scattering in poly (vinyl alcohol) hydrogels reinforced with nanocellulose for ophthalmic use. Opt. Mater. Express 2017, 7, 2824–2837.
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279.
- White, C.J.; Thomas, C.R.; Byrne, M.E. Bringing comfort to the masses: A novel evaluation of comfort agent solution properties. Contact Lens Anterior Eye 2014, 37, 81–91.
- Rah, M.J. A review of hyaluronan and its ophthalmic applications. Optometry 2011, 82, 38–43.
- Stach, S.; Ţălu, Ş.; Trabattoni, S.; Tavazzi, S.; Głuchaczka, A.; Siek, P.; Zając, J.; Giovanzana, S. Morphological Properties of Siloxane-Hydrogel Contact Lens Surfaces. Curr. Eye Res. 2017, 42, 498–505.
- Salzillo, R.; Schiraldi, C.; Corsuto, L.; D’Agostino, A.; Filosa, R.; De Rosa, M.; La Gatta, A. Optimization of hyaluronan-based eye drop formulations. Carbohydr. Polym. 2016, 153, 275–283.
- Johnson, M.E.; Murphy, P.J.; Boulton, M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 109–112.
- Lin, C.-H.; Cho, H.-L.; Yeh, Y.-H.; Yang, M.-C. Improvement of the surface wettability of silicone hydrogel contact lenses via layer-by-layer self-assembly technique. Colloids Surf. B Biointerfaces 2015, 136, 735–743.
- Van Beek, M.; Jones, L.; Sheardown, H. Hyaluronic acid containing hydrogels for the reduction of protein adsorption. Biomaterials 2008, 29, 780–789.
- Paterson, S.M.; Liu, L.; Brook, M.A.; Sheardown, H. Poly(ethylene glycol)-or silicone-modified hyaluronan for contact lens wetting agent applications. J. Biomed. Mater. Res. Part A 2015, 103, 2602–2610.
- Barbault-Foucher, S.; Gref, R.; Russo, P.; Guechot, J.; Bochot, A. Design of poly-ε-caprolactone nanospheres coated with bioadhesive hyaluronic acid for ocular delivery. J. Control. Release 2002, 83, 365–375.
- Moustafa, M.A.; Elnaggar, Y.S.R.; El-Refaie, W.M.; Abdallah, O.Y. Hyalugel-integrated liposomes as a novel ocular nanosized delivery system of fluconazole with promising prolonged effect. Int. J. Pharm. 2017, 534, 14–24.
- Li, C.; Chen, R.; Xu, M.; Qiao, J.; Yan, L.; Guo, X.D. Hyaluronic acid modified MPEG- b -PAE block copolymer aqueous micelles for efficient ophthalmic drug delivery of hydrophobic genistein. Drug Deliv. 2018, 25, 1258–1265.
- Korogiannaki, M.; Guidi, G.; Jones, L.; Sheardown, H. Timolol maleate release from hyaluronic acid-containing model silicone hydrogel contact lens materials. J. Biomater. Appl. 2015, 30, 361–376.
- Korogiannaki, M.; Zhang, J.; Sheardown, H. Surface modification of model hydrogel contact lenses with hyaluronic acid via thiol-ene “click” chemistry for enhancing surface characteristics. J. Biomater. Appl. 2017, 32, 446–462.
- Deng, X.; Korogiannaki, M.; Rastegari, B.; Zhang, J.; Chen, M.; Fu, Q.; Sheardown, H.; Filipe, C.D.M.; Hoare, T. “Click” Chemistry-Tethered Hyaluronic Acid-Based Contact Lens Coatings Improve Lens Wettability and Lower Protein Adsorption. ACS Appl. Mater. Interfaces 2016, 8, 22064–22073.
- Weeks, A.; Morrison, D.; Alauzun, J.G.; Brook, M.A.; Jones, L.; Sheardown, H. Photocrosslinkable hyaluronic acid as an internal wetting agent in model conventional and silicone hydrogel contact lenses. J. Biomed. Mater. Res. Part A 2012, 100 A, 1972–1982.
This entry is offline, you can click here to edit this entry!
