Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Allergy
The concept of cytokine storm has been better elucidated and extended to the pathogenesis of many other conditions, such as sepsis, autoinflammatory disease, primary and secondary hemophagocytic lymphohistiocytosis, and multicentric Castleman disease. Moreover, cytokine storm has recently emerged as a key aspect in the novel Coronavirus disease 2019, as affected patients show high levels of several key pro-inflammatory cytokines, such as IL-1, IL-2, IL-6, TNF-α, IFN-γ, IP-10, GM-CSF, MCP-1, and IL-10, some of which also correlate with disease severity.
- cytokine storm
- COVID-19
1. Clinical Features of the Cytokine Storm
Although manifestations may vary based on the underlying pathology, a cytokine storm typically manifests as an influenza-like syndrome that may evolve or be complicated by multi-organ damage [1][2][3][4]. Fever is almost constant, with very high body temperature in the most severe cases. Other common manifestations include fatigue, headache, arthromyalgia, diarrhea, lymphadenopathy, hepatosplenomegaly, sensory changes, and skin rash. Tachypnea and hypoxemia are often present and can evolve to acute respiratory distress syndrome (ARDS). Acute kidney injury, liver damage, and stress-related cardiomyopathy can also develop in severe patients. Capillary leak syndrome with anasarca and neurological involvement with encephalopathy are also some possible complications of the cytokine storm. Coagulation is impaired, with the possible occurrence of thrombotic phenomena evolving in disseminated intravasal coagulopathy; bleeding risk is also increased at the same time. Laboratory findings in cytokine storm include leukocytosis, leucopenia, anemia, thrombocytopenia, and elevated levels of C-reactive protein (CRP), ferritin, triglycerides, and D-dimer.
2. COVID-19 Related Cytokine Storm
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a member of Coronaviridae, a family of enveloped, positive-sense, single-stranded ribonucleic acid (RNA) viruses which infect humans and other mammals [5]. It is transmitted by air and primarily affects the respiratory system. Previous studies demonstrated that severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS) were both capable of inducing a cytokine storm [6]. Hence, after the advent of SARS-CoV-2, cytokine storm was advocated as a key pathogenetic factor in COVID-19. Numerous studies have shown that COVID-19 patients have increased levels of numerous inflammatory cytokines, including IL-1β, IL-2, IL-6, IL-10, IFN-γ, TNF-α, IFN-γ-inducible protein 10 (IP-10), granulocyte macrophage-colony stimulating factor (GM-CSF), and monocyte chemoattractant protein-1 (MCP-1), and that these cytokines correlate with the disease severity [7][8][9][10][11]. Many studies also demonstrated the presence of inflammatory infiltrates within various tissues in COVID-19 patients, both from bioptic and autoptic samples [12][13][14]. In most cases, the disease consists of a self-limiting flu-like syndrome; however, in predisposed subjects, the infection of lung cells, in particular of type II pneumocytes, can cause the recall of a rich inflammatory cell infiltrate, consisting of neutrophils, macrophages, CD8+ and CD4+ T lymphocytes, and massive production of cytokines, leading to bilateral pneumonia, ARDS, and multi-organ damage [15].
The inadequate immune response to the virus has been proposed as a possible mechanism in the development of cytokine storms during SARS-CoV-2 infection [16][17]. As demonstrated by Blanco-Melo and colleagues, infected cells show an impaired capacity to produce interferons, key mediators for a properly host response against viral infections; at the same time, they produce high levels of neutrophil- and macrophage-recruitant chemokines [18]. Additionally, different research groups have demonstrated that COVID-19 patients produce autoantibodies against several immuno-modulatory proteins and, in particular, the presence of anti-type I interferon antibodies is associated with severe disease and death [19][20]. Therefore, in the first phase of infection, the innate immune system may not be able to efficiently clear the infected cells and, on the contrary, could favor the replication of the virus. Consistently with this hypothesis, Jiadi Lv et al. observed that SARS-CoV-2 survives and replicates inside macrophages [21]. In a second phase, the immune system would recover the ability to effectively fight the virus, but since the latter has been able to replicate undisturbed, it would at that point produce an exaggerated reaction.
3. Targeting the Cytokine Storm in COVID-19
3.1. Anti-Inflammatory/Immunosuppressive Agents
Chloroquine and hydroxychloroquine were widely used at the beginning of the pandemic. They seemed to be promising drugs for the treatment of COVID-19 due to their multiple pharmacological properties. They inhibit the synthesis of cytokines, such as IL-1 and IL-6, but they also enhance the production of other inflammatory mediators [22]. Therefore, chloroquine and hydroxychloroquine have anti-inflammatory effects. Moreover, they showed antiviral activity against a broad spectrum of microorganisms, including SARS-CoV-1. Preliminary in vitro and in vivo findings seemed to confirm their beneficial effect against SAR-CoV-2 infection [23][24]. Nevertheless, data from randomized controlled trials (RCTs) and subsequent metanalysis demonstrated that they have little or no impact on COVID-19 prevention or treatment. Furthermore, they were associated with an increased risk of adverse events [25].
Colchicine is an anti-inflammatory drug used to treat gout, pericarditis, and familial Mediterranean fever, which acts by inhibiting activation and migration of neutrophils and by interfering with the inflammasome complex, essential for IL-1β and IL-18 production [26]. An RCT showed the potential benefit in the treatment of SARS-CoV-2 infection, but further research did not confirm these data [27][28][29]. Therefore, colchicine is not recommended by current guidelines.
3.2. RAAS Targeting Drugs
Based on the hypothesis that the RAAS can contribute to the genesis of the COVID-19-related cytokine storm, ACE inhibitors and sartans have been studied in patients with COVID-19, but their efficacy is still uncertain. While several retrospective studies have shown a potential protective effect, an RCT found no clinical benefit from the use of these drugs in patients with COVID-19 [30][31][32][33][34][35]. Further randomized clinical trials are currently underway and could help clarify the issue (NCT04335786, NCT04311177, NCT04328012). Another possible way to target the RAAS, although not yet tested, could be the use of a direct renin inhibitor, such as the Food and Drug Administration (FDA)-approved aliskiren, to reduce the production of AT1 (Figure 1).
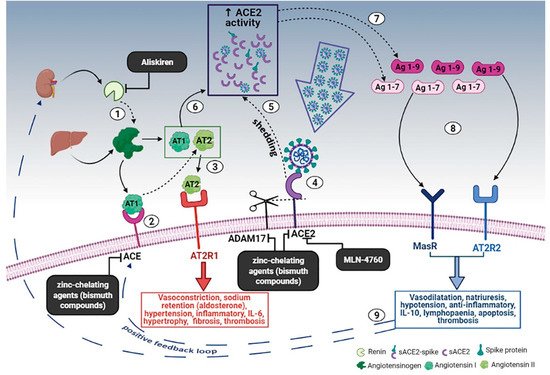
Figure 1. Schematic diagram of the effects of the RAAS system during SARS-CoV-2 infection and the proposed treatment. (1) Renin secreted by the kidney cleaves angiotensinogen, produced by the liver, to form AT1; (2) AT1 is converted to AT2 by pulmonary ACE. (3) AT2 binds to AT2R1 (angiotensin II receptors 1). The excess of AT2 through AT2R1 hyperactivation causes vasoconstriction, sodium retention (by aldosterone release), hypertension, inflammatory, IL-6, hypertrophy, fibrosis, and thrombosis. (4) SARS-CoV-2 binds to ACE2 to enter the host cell; however, the cellular protective response leads to ACE2 shedding. (5) ADAM17-regulated ectodomain shedding of ACE2 results in an increased amount of soluble and active ACE2 (sACE2). (6) AT1 and AT2 can also bind to sACE2. (7) They are then metabolized by ACE2 into Ag 1–9 and Ag 1–7, respectively. (8) The excess of Ag 1–9 and Ag 1–7 signaling via AT2R2 and MasR can induce vasodilatation, natriuresis, hypotension, anti-inflammatory effects, IL-10, lymphopenia, apoptosis, and thrombosis. (9) These events, in turn, produce a compensatory upregulation of both renin secretion and ACE activity, which establish the onset of a positive feedback loop. In the black boxes, drugs that can potentially stop the positive feedback loop by inhibiting enzymes of the RAAS are indicated. Dashed arrows indicate enzymatic activity, full arrows indicate non-enzymatic passage, and dashed blue arrows represent the positive feedback loop. Created in Bio-render.com. Reprinted from Ref. [36].
3.3. Cytokine Inhibitors
With the emergence of biotechnological pharmacology, numerous cytokine inhibitors have been developed and successfully used in immune-rheumatological pathologies, including conditions characterized by cytokine storm syndrome (CSS). Hence, as the cytokine storm could also play a crucial role in the pathogenesis of COVID-19, researchers experimented with the use of several of these drugs in patients infected by SARS-CoV-2. IL-1 is produced following the activation of the innate immune system; it is one of the most important pro-inflammatory molecules involved in the cytokine storm. Anakinra is a recombinant form of the IL-1 receptor antagonist, which, similarly to its endogenous analog, is able to competitively inhibit the binding of IL-1β to its receptor IL1R. This drug has been used successfully in numerous autoimmune and auto-inflammatory conditions, including cytokine release syndrome (CRS) secondary to CAR-T cell therapy and HLH [37][38][39]. These characteristics have led to the experimentation of anakinra in COVID-19 patients with conflicting results. A series of cohort studies recorded a clear benefit from the use of this drug in severe forms of COVID-19 [40][41][42]. On the other hand, a recent RCT conducted in France was suspended prematurely for futility, as no advantage was appreciated in the treatment group [43]. However, the results of the latter study were contested because the sample was composed of patients with mild to moderate forms of the disease, characterized by relatively low levels of C reactive protein, thus excluding the patients who could most benefit from the inhibition of IL-1 (i.e., severe forms characterized by signs and symptoms of CSS).
Rather than targeting a single molecule, Janus kinase (JAK) inhibitors can down-modulate the signaling pathways of multiple cytokines simultaneously: in fact, these agents act by inhibiting JAK, essential components of the Janus kinase-signal transducers and activators of the transcription (JAK-STAT) transduction system which mediate the cellular effects of several cytokine receptors, including IL-2, IL-6, IL-10, IFN-γ, and GM-CSF. JAK inhibitors have aroused particular interest following the positive results of two multicenter, double-blind, placebo-controlled trials.
3.4. Antioxidants
The role of free radicals and oxidative stress in the pathogenesis of COVID-19 has been relatively denied. However, it is known that the recruitment and activation of immune cells in the context of numerous respiratory infections leads to the production of reactive oxygen and nitrogen species. These molecules contribute to the escalation of the inflammatory reaction, stimulating the synthesis of pro-inflammatory cytokines and damaging cell membranes through oxidative mechanisms, thus contributing to the onset of endothelial damage and thrombotic phenomena. In this sense, antioxidant agents could represent an intriguing adjunctive therapy in severe COVID-19 patients. Among others, particularly favorable properties are possessed by methylene blue, a tricyclic phenothiazine, approved as a therapy for methemoglobinemia and malaria, but also tested with success in other pathological conditions, including septic shock [44]. In the hyperinflammatory context of the cytokine storm, methylene blue may be able to simultaneously inhibit the production of reactive oxygen species (ROS), reactive nitrogen oxide species (RNOS), and cytokines themselves, thanks to its ability to counteract the generation of superanions by the xanthine oxidase pathway, to inhibit the synthesis of nitrous oxide (NO) by NO synthase, and to attenuate the nuclear factor-kB (NF-kB) signaling. In addition, in an in vitro study, methylene blue was shown to inhibit cellular infection by SARS-CoV-2 [45]. Currently, methylene blue has only been clinically tested in COVID-19 patients in a small, open-label, randomized trial in Iran.
3.5. Blood Purification Therapies
An alternative, non-pharmacological approach to CSS is represented by blood purification therapies. Previous studies demonstrated that these techniques could remove cytokines from patients’ blood, and therefore, these techniques have been used in the treatment of various immune diseases [46]. In particular, therapeutic plasma exchange (TPE) was applied with promising results in several cytokine-storm-related conditions, such as HLH and sepsis [47][48][49][50]. Clinical results from many case reports, case series, and retrospective studies support TPE as an effective therapy in selected COVID-19 patients [51][52][53][54][55][56], but data from more structured RCTs are still lacking.
3.6. Vaccine and Cytokine Storm
It has been suggested that COVID-19 vaccines may help prevent the cytokine storm through the early control of SARS-CoV-2 infection [57]. However, precise data concerning the prevalence of cytokine storms among vaccinated COVID-19 patients are not available. In the elderly, the “inflammaging” phenomenon may enhance cytokine release in COVID-19, but it may also reduce the immune response to the vaccine, as happens with other vaccines [58][59]. Therefore, the efficacy of COVID-19 vaccines in preventing the cytokine storm may be decreased in older patients [60]. To date, specific research concerning the actual effects of COVID-19 vaccines on the development of the cytokine storm has not been carried out or published. In vaccine clinical trials, researchers usually use disease severity, mortality, infection, transmission, or other surrogate endpoints to evaluate the vaccine efficacy, and the cytokine storm incidence is not studied [61]. Supposedly, the vaccine-induced immune response to the virus should prevent the cytokine storm by reducing the initial inflammatory reaction to the infection. Vaccine efficacy trials demonstrated high percentages of protection against severe disease [62]. However, new virus variants may decrease the vaccine’s effectiveness [62].
This entry is adapted from the peer-reviewed paper 10.3390/medicina58020144
References
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273.
- Murthy, H.; Iqbal, M.; Chavez, J.C.; Kharfan-Dabaja, M.A. Cytokine Release Syndrome: Current Perspectives. ITT 2019, 8, 43–52.
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56.
- Sen, E.S.; Clarke, S.L.N.; Ramanan, A.V. Macrophage Activation Syndrome. Indian J. Pediatr. 2016, 83, 248–253.
- Zanza, C.; Racca, F.; Longhitano, Y.; Piccioni, A.; Franceschi, F.; Artico, M.; Abenavoli, L.; Maiese, A.; Passaro, G.; Volonnino, G.; et al. Risk Management and Treatment of Coagulation Disorders Related to COVID-19 Infection. Int. J. Environ. Res. Public Health 2021, 18, 1268.
- Maiese, A.; Bolino, G.; Mastracchio, A.; Frati, P.; Fineschi, V. An immunohistochemical study of the diagnostic value of TREM-1 as marker for fatal sepsis cases. Biotech Histochem. 2019, 94, 159–166.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Chen, L.; Liu, H.G.; Liu, W.; Liu, J.; Liu, K.; Shang, J.; Deng, Y.; Wei, S. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, E005.
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect Dis. 2020, 95, 332–339.
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643.
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848.
- Manetti, A.C.; Maiese, A.; Paolo, M.D.; De Matteis, A.; La Russa, R.; Turillazzi, E.; Frati, P.; Fineschi, V. MicroRNAs and Sepsis-Induced Cardiac Dysfunction: A Systematic Review. Int J Mol Sci. 2020, 22, 321.
- Maiese, A.; Manetti, A.C.; Bosetti, C.; Del Duca, F.; La Russa, R.; Frati, P.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. SARS-CoV-2 and the brain: A review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021, 31, e13013.
- Maiese, A.; Frati, P.; Del Duca, F.; Santoro, P.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics 2021, 11, 1647.
- Domingo, P.; Mur, I.; Pomar, V.; Corominas, H.; Casademont, J.; de Benito, N. The four horsemen of a viral Apocalypse: The pathogenesis of SARS-CoV-2 infection (COVID-19). EBioMedicine 2020, 58, 102887.
- Maiese, A.; Passaro, G.; De Matteis, A.; Fazio, V.; La Russa, R.; Di Paolo, M. Thromboinflammatory response in SARS-CoV-2 sepsis. Med. Leg. J. 2020, 88, 78–80.
- Frisoni, P.; Neri, M.; D’Errico, S.; Alfieri, L.; Bonuccelli, D.; Cingolani, M.; Di Paolo, M.; Gaudio, R.M.; Lestani, M.; Marti, M.; et al. Cytokine storm and histopathological findings in 60 cases of COVID-19-related death: From viral load research to immunohistochemical quantification of major players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci. Med. Pathol. 2021, 31, 1–15.
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9.
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288.
- Bastard, P.; Gervais, A.; Le Voyer, T.; Rosain, J.; Philippot, Q.; Manry, J.; Michailidis, E.; Hoffmann, H.H.; Eto, S.; Garcia-Prat, M.; et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci. Immunol. 2021, 6, eabl4340.
- Lv, J.; Wang, Z.; Qu, Y.; Zhu, H.; Zhu, Q.; Tong, W.; Bao, L.; Lv, Q.; Cong, J.; Li, D.; et al. Distinct uptake, amplification, and release of SARS-CoV-2 by M1 and M2 alveolar macrophages. Cell Discov. 2021, 7, 24.
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases. Lancet Infect. Dis. 2003, 3, 722–727.
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271.
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73.
- Singh, B.; Ryan, H.; Kredo, T.; Chaplin, M.; Fletcher, T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst. Rev. 2021, CD013587.
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350.
- Deftereos, S.G.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.D.; Giotaki, S.G.; Gargalianos, P.; Metallidis, S.; Sianos, G.; Baltagiannis, S.; Panagopoulos, P.; et al. GRECCO-19 investigators. Effect of Colchicine vs. Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2013136.
- Tardif, J.C.; Bouabdallaoui, N.; L’Allier, P.L.; Gaudet, D.; Shah, B.; Pillinger, M.H.; Lopez-Sendon, J.; da Luz, P.; Verret, L.; Audet, S.; et al. For the COLCORONA Investigators. Colchicine for community-treated patients with COVID-19 (COLCORONA): A phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir. Med. 2021, 9, 924–932.
- RECOVERY Collaborative Group. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet Respir. Med. 2021, 9, 1419–1426.
- Liu, Y.; Huang, F.; Xu, J.; Yang, P.; Qin, Y.; Cao, M.; Wang, Z.; Li, X.; Zhang, S.; Ye, L.; et al. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv 2020.
- Yang, G.; Tan, Z.; Zhou, L.; Yang, M.; Peng, L.; Liu, J.; Cai, J.; Yang, R.; Han, J.; Huang, Y.; et al. Effects of Angiotensin II Receptor Blockers and ACE (Angiotensin-Converting Enzyme) Inhibitors on Virus Infection, Inflammatory Status, and Clinical Outcomes in Patients With COVID-19 and Hypertension: A Single-Center Retrospective Study. Hypertension 2020, 76, 51–58.
- Meng, J.; Xiao, G.; Zhang, J.; He, X.; Ou, M.; Bi, J.; Yang, R.; Di, W.; Wang, Z.; Li, Z.; et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect 2020, 9, 757–760.
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of COVID-19. N. Engl. J. Med. 2020, 382, 2441–2448.
- Mehra, M.R.; Desai, S.S.; Kuy, S.; Henry, T.D.; Patel, A.N. Cardiovascular Disease, Drug Therapy, and Mortality in COVID-19. N. Engl. J. Med. 2020, 382, e102.
- Cohen, J.B.; Hanff, T.C.; William, P.; Sweitzer, N.; Rosado-Santander, N.R.; Medina, C.; Rodriguez-Mori, J.E.; Renna, N.; Chang, T.I.; Corrales-Medina, V.; et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: A prospective, randomised, open-label trial. Lancet Respir. Med. 2021, 9, 275–284.
- Zanza, C.; Tassi, M.F.; Romenskaya, T.; Piccolella, F.; Abenavoli, L.; Franceschi, F.; Piccioni, A.; Ojetti, V.; Saviano, A.; Canonico, B.; et al. Lock, Stock and Barrel: Role of Renin-Angiotensin-Aldosterone System in Coronavirus Disease 2019. Cells 2021, 10, 1752.
- Miettunen, P.M.; Narendran, A.; Jayanthan, A.; Behrens, E.M.; Cron, R.Q. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: Case series with 12 patients. Rheumatology 2011, 50, 417–419.
- Durand, M.; Troyanov, Y.; Laflamme, P.; Gregoire, G. Macrophage activation syndrome treated with anakinra. J. Rheumatol. 2010, 37, 879–880.
- Sönmez, H.E.; Demir, S.; Bilginer, Y.; Özen, S. Anakinra treatment in macrophage activation syndrome: A single center experience and systemic review of literature. Clin. Rheumatol. 2018, 37, 3329–3335.
- Huet, T.; Beaussier, H.; Voisin, O.; Jouveshomme, S.; Dauriat, G.; Lazareth, I.; Sacco, E.; Naccache, J.M.; Bézie, Y.; Laplanche, S.; et al. Anakinra for severe forms of COVID-19: A cohort study. Lancet Rheumatol. 2020, 2, e393–e400.
- Cavalli, G.; De Luca, G.; Campochiaro, C.; Della Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Boffini, N.; et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e325–e331.
- Aouba, A.; Baldolli, A.; Geffray, L.; Verdon, R.; Bergot, E.; Martin-Silva, N.; Justet, A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: Case series. Ann. Rheum. Dis. 2020, 79, 1381–1382.
- CORIMUNO-19 Collaborative Group (2021). Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): A randomised controlled trial. Lancet Respir. Med. 2021, 9, 295–304.
- Scigliano, G.; Scigliano, G.A. Methylene blue in covid-19. Med. Hypotheses 2021, 146, 110455.
- Bojadzic, D.; Alcazar, O.; Buchwald, P. Methylene Blue Inhibits the SARS-CoV-2 Spike–ACE2 Protein-Protein Interaction–a Mechanism that can Contribute to its Antiviral Activity Against COVID-19. Front. Pharmacol. 2021, 11, 600372.
- Atan, R.; Crosbie, D.C.A.; Bellomo, R. Techniques of extracorporeal cytokine removal: A systematic review of human studies. Ren. Fail. 2013, 35, 1061–1070.
- Demirkol, D.; Yildizdas, D.; Bayrakci, B.; Karapinar, B.; Kendirli, T.; Koroglu, T.F.; Dursun, O.; Erkek, N.; Gedik, H.; Citak, A.; et al. Turkish Secondary HLH/MAS Critical Care Study Group. Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: What is the treatment? Crit. Care 2012, 16, R52.
- Bosnak, M.; Erdogan, S.; Aktekin, E.H.; Bay, A. Therapeutic plasma exchange in primary hemophagocytic lymphohistiocytosis: Reports of two cases and a review of the literature. Transfus. Apher. Sci. 2016, 55, 353–356.
- Lorenz, G.; Schul, L.; Schraml, F.; Riedhammer, K.M.; Einwächter, H.; Verbeek, M.; Slotta-Huspenina, J.; Schmaderer, C.; Küchle, C.; Heemann, U.; et al. Adult macrophage activation syndrome-haemophagocytic lymphohistiocytosis: ‘of plasma exchange and immunosuppressive escalation strategies’—A single centre reflection. Lupus 2020, 29, 324–333.
- Rimmer, E.; Houston, B.L.; Kumar, A.; Abou-Setta, A.M.; Friesen, C.; Marshall, J.C.; Rock, G.; Turgeon, A.F.; Cook, D.J.; Houston, D.S.; et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: A systematic review and meta-analysis. Crit. Care 2014, 18, 699.
- Truong, A.D.; Auld, S.C.; Barker, N.A.; Friend, S.; Wynn, A.T.; Cobb, J.; Sniecinski, R.M.; Tanksley, C.L.; Polly, D.M.; Gaddh, M.; et al. Therapeutic plasma exchange for COVID-19-associated hyperviscosity. Transfusion 2021, 61, 1029–1034.
- Kamran, S.M.; Mirza, Z.E.; Naseem, A.; Liaqat, J.; Fazal, I.; Alamgir, W.; Saeed, F.; Saleem, S.; Nisar, S.; Yousaf, M.A.; et al. Therapeutic plasma exchange for coronavirus disease-2019 triggered cytokine release syndrome; a retrospective propensity matched control study. PLoS ONE 2021, 16, e0244853.
- Dogan, L.; Kaya, D.; Sarikaya, T.; Zengin, R.; Dincer, A.; Akinci, I.O.; Afsar, N. Plasmapheresis treatment in COVID-19–related autoimmune meningoencephalitis: Case series. Brain Behav. Immun. 2020, 87, 155–158.
- Keith, P.; Day, M.; Choe, C.; Perkins, L.; Moyer, L.; Hays, E.; French, M.; Hewitt, K.; Gravel, G.; Guffey, A.; et al. The successful use of therapeutic plasma exchange for severe COVID-19 acute respiratory distress syndrome with multiple organ failure. SAGE Open Med. Case Rep. 2020, 8, 2050313X20933473.
- Morath, C.; Weigand, M.A.; Zeier, M.; Speer, C.; Tiwari-Heckler, S.; Merle, U. Plasma exchange in critically ill COVID-19 patients. Crit. Care 2020, 24, 481.
- Adeli, S.H.; Asghari, A.; Tabarraii, R.; Shajari, R.; Afshari, S.; Kalhor, N.; Vafaeimanesh, J. Therapeutic plasma exchange as a rescue therapy in patients with coronavirus disease 2019: A case series. Pol. Arch. Intern. Med. 2020, 130, 455–458.
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632.
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect Dis. 2012, 12, 655.
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021, 170, 1–25.
- Ciabattini, A.; Garagnani, P.; Santoro, F.; Rappuoli, R.; Franceschi, C.; Medaglini, D. Shelter from the cytokine storm: Pitfalls and prospects in the development of SARS-CoV-2 vaccines for an elderly population. Semin. Immunopathol. 2020, 42, 619–634.
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021, 21, e26–e35.
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636.
This entry is offline, you can click here to edit this entry!
