Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
In comparison to the undoped carbon nanomaterials, heteroatoms such as nitrogen-, sulphur-, boron-, phosphorous-, etc., incorporated nanomaterials have become well-accepted as potential electrocatalysts in water splitting, supercapacitors and dye-sensitized solar cells.
- carbon nanomaterials
- nitrogen doping
- sulphur doping
- co-doping
1. Nitrogen-Doped Metal-Free Carbon Nanostructured Electrocatalysts
1. Nitrogen-Doped Carbon Nanotube Electrocatalysts
The functionalized nanotubes garnered significant attention in the field of the reinforced and conductive plastics, sensing materials and photovoltaic materials as scanning probe microscopy tips and many more applications. There have been two broad methods to synthesize substituted N-doped CNTs: (a) in situ process for insertion of nitrogen atom into the CNTs during the reaction, only [74,75,76,77,78]; and (b) postfunctionalization of CNTs with nitrogen by using various precursors and compounds like organic moieties. However, the postfunctionalization method has not been well investigated until now [79,80,81]. These nanomaterials have also been synthesized using other potential synthetic strategies, viz., arc discharge, laser ablation and plasma etching [82,83,84,85,86,87]. However, these methodologies required higher temperature conditions and a limited type of nitrogen or carbon precursors. Moreover, rapid evaporation of precursors and application of nitrogen or ammonia atmosphere has been required. In the chemical vapor deposition (CVD) method, the process could completed at a lower temperature range with and without the presence of an organometallic catalyst and by using a wide range of carbon or nitrogen precursors. This method could produce 20–25 g of N-carbon nanotubes per gram of catalyst, and nitrogen atoms are embedded into the hexagonal carbon network at various ratios with 10 atoms [81]. In the literature, nitrogen incorporation has been reported with nitrogen contents of <1 atom% to 20 atom% [75,88,89]. Highly oriented nanotubes with regular diameter and bond-length were termed in literature as “carpet-like” structures [90]. In this work, nitrogen was incorporated into the already synthesized CNT structure; however, this synthesis method was depicted as highly complex and tedious with multistep techniques. The first step is initiated with a chemical oxidation process of tips or structural defects of CNTs, followed by coupling with other molecules through carboxylic, carbonyl and/or hydroxyl groups. The covalent functionalization via bond formation to the π-conjugated structure of CNT leads to the rehybridization of sp2 bond to sp3. In this type of structure, nitrogen is attached to carbon following two different manners: (a) pyridine-type nitrogen, in which each nitrogen atom is bonded to two different carbon atoms, leading to the formation of cavities within the side wall of the tube, and (b) substitution N, in which a nitrogen atom bonds with three C atoms, as presented in Figure 2. Nitrogen contains an additional electron in its structure, in comparison to the carbon network; therefore, a nitrogen-incorporated CNT structure usually exhibits metallic properties [90,91,92]. The nitrogen group can also enhance the reactivity on the graphene in comparison to the pure CNT structures, which results in the potential applications of these materials in fast-responsive sensing technology; as effective field-emissions sources; and as polystyrene, epoxy composites, protein and nanoparticle immobilizers [78,93,94,95,96]. The most popular covalent functionality, with the application of plasma etching or by HNO3/H2SO4 treatment, includes carbonyl or carboxyl groups [97].
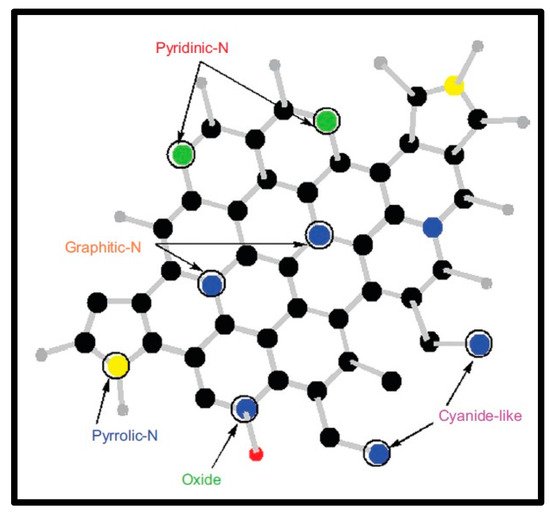
Figure 2. Nitrogen doping in the carbon nanomaterial structures (Reproduced with permission from S. Majeed et al. [89]).
The plasma etching technique is essentially applicable during functionalization processes in a nitrogen atmosphere. In the next step, carboxyl groups are acylated with thionyl chloride to establish a basis for different amine compounds [98] or to combine with DNA and proteins [99,100]. The noncovalent functionalization is mostly conducted by adsorption or through the wrapping of the CNTs in polymer polynuclear aromatic compounds, surfactants or biomolecules by Vander Waals forces and π–π interactive forces. Other synthetic approaches of CNTs include the arc evaporation method of graphite [82,101]. The noncovalent methods are more favourable over covalent, as the chemical functionalization can be performed on the CNTs without affecting their structures and electronic networks on the nanotubular structures.
1.2. Chemical Vapour Deposition (CVD) Method
Chemical vapour deposition (CVD) is a technique to synthesize carbon nanotubes in bulk amounts, which involves the pyrolysis of different organic molecules, viz., CH4, C6H6, C2H2, etc., in inert atmosphere over Ni, Co, Fe, etc., catalysts [102,103]. Due to the simplicity and cost-effectiveness of CVD, researchers prefer to follow this methodology during the functionalization process.
In 1997, Dai et al. introduced H2O plasma etching technology to generate surface patterns of polar groups with oxygen [104]. This methodology was further followed by Yu et al. [86] to develop SiO2 nanoparticles as the metal-free catalysts, in which a SiO2/Si wafer with a 30 nm-thick SiO2 coating was employed with H2O plasma etching at 30 W, 250 kHz and 0.62 Torr for 20 min. This plasma-etched substrate was placed into a tube furnace for the synthesis of CNTs by using the CVD method. Figure 3 represents the schematic diagram to represent the growth of CNTs. These materials acted as potential electrocatalysts in an oxygen reduction reaction analysed in 0.5 M H2SO4 solution saturated with N2 or O2. Figure 4 shows the various electrochemical studies conducted in this work. All the electrocatalytic studies have shown excellent results and long-term stability in an acidic medium in comparison to undoped CNTs. The authors also claimed that, due to the highly generic nature of the plasma etching technique, this synthetic strategy can be well accepted in various fields, from energy applications to electronic and biomedical systems [86]. Kim et al. mentioned a similar synthesis process in an Ar atmosphere at 800 °C for 1 h duration in which ferrocene, pyridine or ethylenediamine used as a catalyst carbon and nitrogen precursor, respectively [50]. TEM images of bamboo-structured N-doped CNTs (NCNTs) are presented in Figure 5. These products were used as excellent electrocatalysts in ORR of fuel cell applications. The same research group reported synthesis of nitrogen-doped CNTs by following a single step the CVD method in which either ferrocene or iron (II) phthalocyanine was used as the catalyst and pyridine as the carbon and nitrogen precursor, respectively. These materials have also used successfully as ORR electrocatalysts [105].
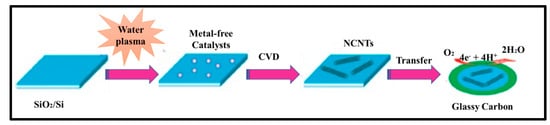
Figure 3. N-doped metal-free CNTs as ORR electrocatalysts (Reproduced with permission from Chen et al. [86]).
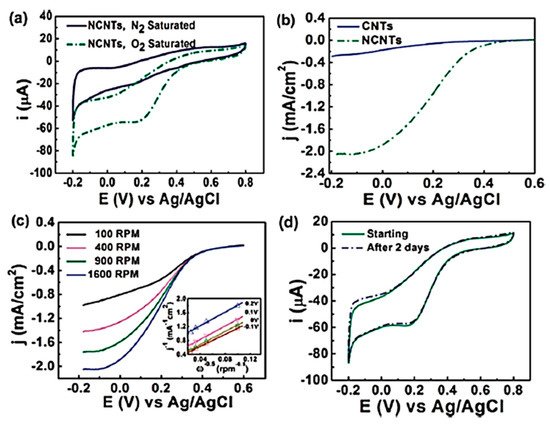
Figure 4. Electrochemical studies in ORR under acidic medium with N-doped metal – free CNTs (Reproduced with permission from Yu et al. [86]).
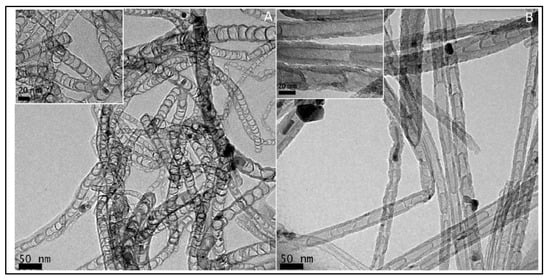
Figure 5. TEM images of bamboo-structured NCNTs (A) ethylenediamine–NCNTs (B) Pyridine–NCNTs (Reproduced with permission from Chen et al. [50]).
In 2011, Feng et al. [52] reported N-doped CNTs to be effective electrocatalysts in microbial fuel cells (MFCs), boasting cost-effectiveness and long durability. Moreover, these materials were depicted as more effective cathodic catalysts than the commonly used platinum catalyst with a maximum power density value of 1600 ± 50 mW·m−2. These N-doped CNTs have shown a lower drop in the percentage of power density than that of Pt/C over 25 cycles. Another research group reported the CVD synthesis floating catalyst method of nitrogen-doped carbon nanotubes using ferrocene/aniline together with toluene as an added carbon source [106]. Yang et al. synthesized aligned nitrogen-doped CNT bundles over 700–800 °C by taking ammonium-exchanged zeolite-β as the substrate material, ferric nitrate as the catalyst and acetonitrile as the carbon precursor [107]. In the same year, He et al. reported controllable synthesis of aligned CNx with a large surface area by pyrolyzing CH3CN/Fe(C5H5)2 on SiO2 and Si substrates over the temperature range of 750–900 °C. The specific diameters of CNTs diminished on Si substrates in comparison to a well-documented rise with temperature on silica, as the growth process followed different mechanisms of formation of catalyst particles [108]. Kim et al. developed N-doped, double-walled CNTs using chemical vapor deposition in which a CH4/NH3/Ar mixture flowed with the rate of 50/10/500 sccm on MgO-supported catalyst powders at the temperature of 850 °C for 10–30 min. of duration [109]. The synthesized CNTs are formed with a diameter of 10–20 nm. The SEM and HRTEM images of N-doped double walled-CNTs presented in Figure 6.
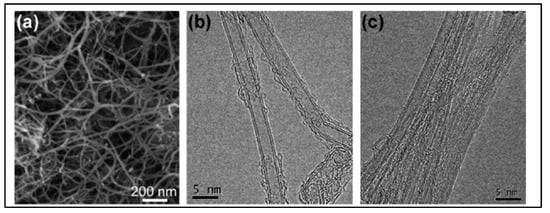
Figure 6. (a) SEM image shows the nanotube bundles with a diameter of 10–20 nm (b) HRTEM image reveals that the bundles are consisted mainly of double walled CNTs DWNTs. (c) A magnified image showing the outer diameter in the range of 1.5–2 nm (Reproduced with permission from Kim et al. [109]).
In recent years, Li et al. reported a one-step CVD method to synthesize three-dimensional nitrogen-doped CNT/graphene hybrid material on nickel foam [110]. In this study, nickel foam and melamine were mixed with the mass ratio of 1:5 kept in a horizontal quartz tube reactor and heated to within the temperature range of 600–800 °C in a hydrogen atmosphere for around 20 min at a flow rate of 70 sccm. In 2020, another research group mentioned a two-step synthetic strategy to develop nitrogen-doped carbon nanotubes derived from g-C3N4 [111]. In this case, exfoliated graphitic carbon nitride was functionalized with nickel oxides and placed in a ceramic boat to keep in the tubular furnace at 900 °C in a nitrogen atmosphere. Hydrogen was introduced for 3 h in the first step and ethylene for 10 min for the reduction process. The synthesis of N-doped MWCNTs with straight structure was reported by Xu et al. by using phthalocyanine derivatives [112] and the mixture of ethylene/hydrogen and ammonia at around 680 °C in a presence of alumina-supported iron catalysts in a CVD furnace [113]. The amount of nitrogen incorporated into CNT can be controlled by using different amounts of nitrogen precursors [80,114]. The rate in which nanotubes grow during synthesis can be enhanced with the increase in its precursor significantly, resulting into the increase in intensity ratio of the D to G bands in Raman spectra. The inner structure of N-doped CNTs constitutes regular morphological transformation from the straight and smoother walls (0 atom% N) to 1.5 atom% N-containing, bamboo-structured CNTs; further, it changed to corrugated structures with 3.1 atom% and higher nitrogen [115]. It has been analysed by Wang et al. that, during the synthesis of N-doped CNTs, when melamine is used as the C/N initiator, it can incorporate 20 atom% nitrogen. In this type of synthesis method, the N atom present in the reaction medium self-assembled with gaseous carbon without any assistance from metal [116]. These N-doped CNTs were utilized successfully as ORR electrocatalysts in methanol fuel cells measured in alkaline media. Figure 7 shown the various electrochemical studies of NCNT/GC.
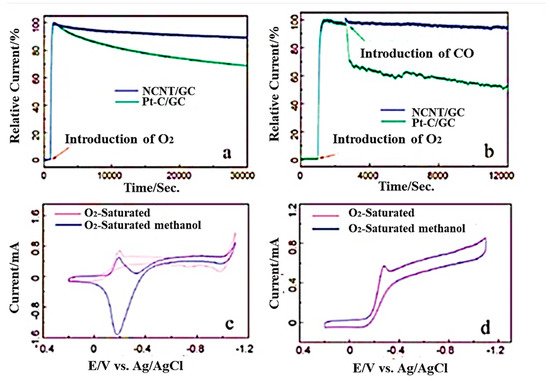
Figure 7. (a) Chronoamperometric studies with NCNT/GC and Pt–C/GC electrodes in oxygen saturated 0.1M KOH. (b) Responses after the introduction of 10% CO. (c) Cyclic voltammograms of Pt/GC and (d) cyclic voltammograms of NCNT/GC electrodes oxygen saturated 0.1 M KOH, with and without 3 M methanol solution (Reproduced with permission from Wang et al. [116]).
The incorporation of nitrogen atoms usually shows very strong ability to promote the self-assembled CNTs. Nitrogen could create highly active sites in carbon networks, which results in remarkable electrocatalytic performance comparable to traditional Pt-based materials as electrocatalysts. Their high activity with excellent stability and selectivity always made N-doped CNTs better electrocatalysts in this purpose. These materials were also strongly resistant to CO poisoning, had a robust structure and were economically favourable. Due to doping in CNT structures, a basic shape could be transformed from a hollow cylinder to a bamboo-shaped structure. The resultant doped materials contained plenty of compartments, the lengths of which gradually decreased with variation in N concentration [117].
1.3. Chemical and Electrochemical Modification Method
The chemical modification methods to synthesize nitrogen-doped CNTs included two different approaches, viz., covalent and noncovalent. During the covalent modification, oxygen-containing functional groups, viz., carboxyl and hydroxyl, were formed and generated on the surface. Among the functional groups, carboxylic acid groups were chosen as the best options, as they could easily proceed a variety of reactions in the modification process and could be easily developed using different oxidizing treatments, e.g., ozonolysis, sonication in nitric and sulfuric acid, refluxing in nitric acid, etc. In the next step, carboxyl-functionalized CNTs were grafted with the functional moieties by using the terminal oxidation process following various mechanisms from the defect site, from chemistry oxidation reactions and esterification/amidation processes to the already oxidized CNTs [118,119], mechanochemical modification [120,121], ionic liquids, cycloaddition reactions [122,123], electrochemical modification reactions, diazotization [124] and radical additions [125].
The efficient and successful doping and tailoring technologies in CNTs involved the controlling of redox properties of the dopant. Nitrogen-doped CNTs have excellent electrocatalytic activity compared to Pt electrodes, which could be acclaimed by the formation of additional active sites on the surface of the materials and has led to better dispersion of the Pt particles over the N–CNT and performed better in methanol oxidation [126]. From the results, it was analysed that doped CNTs as electrode materials always enhanced the output power of the thermoelectrochemical cells. Doping enhanced the electrochemical active surface area (ESCA) values in the CNT electrodes in proportional way. Wei et al. reported doped CNTs mixed with glutaraldehyde functionalized chitosan (GCS), which depicted an improved biocompatibility and higher conductivity in enzyme immobilization process, due to the enhanced kinetics from the N–CNTs [127]. The electrochemical modification process was carried out through two types of coupling reactions, working under oxidative or reductive conditions. In 2002, Kooi et al. worked on anodic coupling reaction to the SWCNTs by using two different aromatic amines, viz., 4-aminobenzylamine and 4-aminobenzoic acid [128]. The noncovalent functionalization process could be carried out through the porphyrin assembled on the N-doped MWCNTs via the Fe-N coordination. Tu et al. reported this noncovalent modification by porphyrin, which led the MWNTs insoluble in water, however, performed well as catalysts and biosensors [129].
1.4. Nitrogen-Doped Carbon Hollow Spheres
The carbon spheres usually referred to the spherical shaped carbon in semicrystalline or crystalline form, and constituted solid, hollow or core-shell morphological structures. Researchers have paid huge attention to nitrogen-doped hollow spherical structures in recent years due to their lower density, greater surface area values, better electrical conductivity, and excellent structural stability. In 2012, Zhu et al. developed a hierarchical porous hollow carbon nanospheres as an oxygen reduction electrocatalyst for zinc–air batteries, which contained active pyridinic-N and graphitic-N by using polystyrene spheres and aniline as the corresponding template and precursor [130]. Gu et al. reported N-doped porous carbon spheres with excellent porosity characteristics, which was used as a potential electrocatalyst in ORR. The unique spherical structures with remarkable stability and recyclability made these materials the most promising ORR electrocatalysts [131]. Hydrothermal carbonization method was adopted to make these materials by using biomass glucose, followed by treatment in ammonia and by subsequent activation treatment. Another research group reported the development of N-doped carbon nanodots @ nanospheres, which were applied as efficient electrocatalyst in ORR, in which high electrocatalytic activity was shown with an onset potential of −0.08 V, and they showed greater durability and greater resistance to the methanol cross-over effect; these results were comparable to commercially available Pt/C electrocatalyst. These N-doped carbon nanodots of sizes 2–6 nm were successfully formed by using the hydrothermal method from natural biomass (e.g., fresh grass) at 180 °C for 10 h duration. Furthermore, these carbon nanodots were subsequently immobilized onto functionalized microporous carbon nanospheres (MCNSs) with an average diameter of ∼100 nm and a surface area of 241 m2 g−1 via a simple hydrothermal process to self-assemble a carbon-based nanocomposite (N-CNDs@MCNSs) in the presence of oxygen (O)-containing surface functional groups [132]. Today, a significant number of research works were carried out on nitrogen encapsulation on metal/metal oxides/carbon nanosphere materials potentially applied as electrodes or electrocatalysts [133,134,135,136,137,138,139,140].
1.5. Nitrogen-Doped Graphene Electrocatalysts
Graphene has been two-dimensionally structured with sp2 hybridized carbon with interesting physical and chemical characteristics. To achieve the desired performance in electrochemical and biochemical applications, nitrogen-enriched graphene materials were synthesized using a wide range of methodologies [141,142,143,144,145,146,147,148,149,150,151]. In 2011, Zhang et al. developed N-doped graphene by thermally annealing graphene oxide in the presence ammonia [152]. Another research group reported a facile and catalyst-free method to develop large-scale synthesis of nitrogen-doped graphene with 10.1 wt% nitrogen content by using the economically favourable industrial material melamine as the nitrogen source [153]. Sheng et al. synthesized nitrogen-doped graphene using the solvothermal method with the reaction between tetrachloromethane with lithium nitride under mild conditions [154]. Figure 8 and Figure 9 present the schematic diagram to synthesize these materials and their potential electrocatalytic applications in ORR under alkaline media, respectively. Another simple way to produce N-doped graphene nanosheets following the solvothermal route is via the reaction between graphene oxide and urea with a nitrogen content of 10.13 atom% [155]. Temperature played a pivotal role in the solvothermal process during the doping of nitrogen in the graphene network [152,153]. Another research group developed pyrrolic and pyridinic type nitrogen incorporation in a graphene structure at 300 and 500 °C, respectively, with the annealing treatment of graphene oxide in the presence of glycine and AgNO3 [156]. This particular methodology produced N-doped graphene with 13.5 atom% of nitrogen into the materials. The CVD method was also adopted by using methane and ammonia; these materials were utilized as metal-free electrocatalysts in ORR applied in fuel cells [157]. Many research groups have also applied the arc discharge method in H2 and He atmosphere and under pyridine vapour to produce the nitrogen-doped graphene structure [158,159].
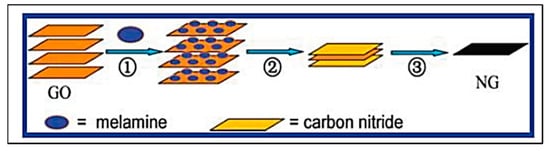
Figure 8. Schematic diagram of nitrogen doping method with melamine in the GO layer (Reproduced with permission from Sheng et al. [154]).
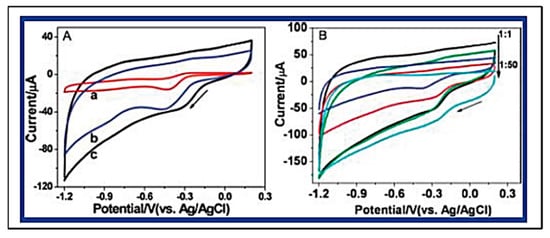
Figure 9. (A) Typical cyclic voltammograms (CVs) for ORR obtained at a bare GCE (a), graphene/GCE (b), and NG5/GCE (N% = 7.1%) (c) in O2 saturated 0.1 M KOH aqueous solution. (B) CVs for ORR at NGs, synthesized with different mass ratio of GO and melamine (1:1, 1:2, 1:5, 1:10, 1:50) at 800 °C, modified GCE in O2 saturated 0.1 M KOH aqueous solution. (Reproduced with permission from Sheng et al. [154]).
Yang et al. reported synthesis of N-doped graphene, the result of which was successfully demonstrated as highly efficient metal-free bifunctional electrocatalysts in the oxygen reduction and evolution reaction [160]. In this report, e- donating quaternary nitrogen sites were responsible for ORR; in contrast, e- withdrawing pyridinic nitrogen acted as active sites in OER, resulting into greater transports of electrons and electrolytes [160]. The schematic diagram to synthesize N-doped graphene nanoribbon networks shown in Figure 10 [160].
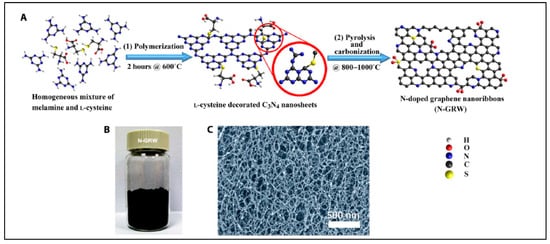
Figure 10. Synthesis of N-doped graphene nanoribbon networks (GRW). (A) Synthesis steps: (1) polymerization at 600 °C for 2 h, and (2) pyrolysis and carbonization at 800° to 1000 °C. (B) Digital photograph of the as-synthesized N-GRW. (C) Scanning electron microscopy (SEM) image of the as-synthesized N-GRW (Reproduced with permission from Yang et al. [160]).
Liu et al. synthesized pyrrolic–nitrogen doped graphene, which was successfully adapted as carbon-free electrocatalysts in electrocatalytic reduction of carbon dioxide to formic acid in their comparative study with the computational method [161]. Earlier, Ju et al. developed nitrogen-doped graphene nanoplatelets as potential metal-free, counter-electrode materials used in organic dye-sensitized solar cells [162]. Rahsepar et al. followed a hybrid hydrothermal-microwave process to synthesize N-doped graphene, which exhibited remarkable electrocatalytic activity in ORR [163]. The number of catalytic sites was enhanced due to the incorporation of N-atom into graphene. Maouche et al. developed nitrogen-doped graphene with porous structures, which was successfully employed as an ORR electrocatalyst [164]. In this work, a facile fabrication technology was carried out with graphitic carbon nitride (g-C3N4) and graphene oxide (GO) as raw materials. Another research group utilized N-doped graphene as an electrocatalyst in ORR under alkaline medium and in anion exchange membrane fuel cells [165].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27030670
This entry is offline, you can click here to edit this entry!
