Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Flexible sensors have improved upon the current clinical arrhythmia detection methods by following the topography of skin and reducing the natural interface mismatch between cardiac monitoring sensors and human skin. Flexible bioelectric, optoelectronic, ultrasonic, and mechanoelectrical sensors have been demonstrated to provide essential information about heart-rate variability, which is crucial in detecting and classifying arrhythmias.
- arrhythmia detection
- cardiovascular monitoring
- soft biosensors
- wearable sensors
- flexible electronics
1. Introduction
Arrhythmia is the presence of abnormal cardiac rhythms. In 2018, more than 500,000 American deaths included arrhythmia as a contributing factor, demonstrating its deleterious impact on patient health [1]. Arrhythmias occur when the electrical pulses of the heart are not functioning properly, causing the heart to beat either too fast, too slow, or skip beats. Impulse-production arrhythmias can be grouped into six categories: premature beats, non-sinus rhythm, fibrillation, tachycardias, bradycardias, and flutter. Premature beats are abnormally timed beats that occur before the sinus rhythm and are caused by the heart being unable to fill with the appropriate amount of blood [2]. Atrial fibrillation, the most common arrhythmia, occurs when the electrical pulses between the upper chambers of the heart, the atria, do not sync with the pulses in the lower chambers of the heart, the ventricles. Ventricular fibrillation, on the other hand, occurs when there is a mismatch between the right and left atria, which makes the heart unable to pump blood to the body [2]. Tachycardias occur when the heart is beating too fast, generally more than 100 beats per minute, and bradycardias occur when the heart is beating too slow, generally less than 40 beats per minute. In addition, impulse-conduction arrhythmia types include atrioventricular block, bundle branch block, Wolff—Parkinson—White syndrome, and escape beats [3]. An atrioventricular block occurs when the impulses between the atria and ventricles become blocked due to a failure in the heart’s conduction system. Bundle branch block occurs as a result of blockages in the pathways in the heart. Wolff—Parkinson—White syndrome occurs when additional electrical pathways are made between the atria and ventricles, resulting in a rapid heartbeat [4].
Arrhythmias have traditionally been diagnosed by medical professionals based on qualitative data, a patient’s medical history, and clinical examinations. Electrocardiography (ECG) has proven instrumental in identifying arrhythmias. The importance of continuous monitoring for specific arrhythmias has been increasingly identified, as both asymptomatic arrhythmias and paroxysmal diseases remain difficult to detect through intermittent clinical ECG recordings [5][6]. The 12-lead Holter monitor has long been the clinical standard for detection and diagnosis of heart-rate diseases using long-term monitoring of ECG [7]. Though these devices are widely used, they are prone to poor patient compliance because of their bulkiness and reliance on wired leads [8]. In addition, these devices experience signal deterioration over time due to the drying of the conductive gels [9]. The advent of miniaturized, one-lead devices has offered an alternative to multi-lead ECG devices. However, they are susceptible to motion artifacts that disrupt data collection [10].
Flexible devices have emerged as alternatives to these rigid devices, eliminating motion artifacts by increasing sensor-to-skin adhesion. Recently, new areas of research have been developed to make these heart-rate monitoring devices cheaper and faster to manufacture, expanding accessibility for these previously costly devices. Additionally, new alternatives to ECG can provide information that ECG alone cannot. For instance, photoplethysmography, ultrasound, seismocardiography, and ballistocardiography can characterize the heart’s electromechanics. Figure 1 shows these soft-sensor types, along with the desirable qualities of the devices. New arrhythmia-detection methodologies also offer more accurate, automatic information to patients for a low cost. For example, deep neural networks, which are capable of learning important features and patterns without extensive preprocessing or feature engineering, are becoming extremely accurate in predicting types of arrhythmia [11].
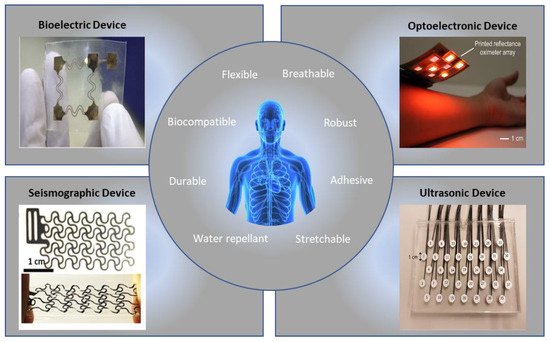
Figure 1. Examples of flexible sensors and functions for accurate arrhythmia detection [12][13][14][15]. (Figures are adapted or reprinted, clockwise, from the top–left: (1) Sensors Actuators A Phys. 2018, 272, 92–101, Copyright 2019, Elsevier; (2) Proc. Natl. Acad. Sci. 2018, 115, E11015–E11024, Copyright 2018, National Academy of Sciences; (3) Creative Common License by MDPI; (4) Creative Common License by Wiley.
2. Bioelectric Signals
2.1. Mechanics of ECG
ECG is the practice of measuring the heart’s electrical activity using pairs of electrodes on the skin. Clinically, this is achieved with 12 leads using 10 electrodes to measure cardiac signals from many angles [16]. A healthy ECG cycle typically consists of five different waves: P, Q, R, S, and T.
2.2. Materials for Flexible ECG Devices
Many ECG electrodes currently used in clinics are made up of three parts: (1) a conductive metal, traditionally Ag/AgCl electrodes; (2) a conductive gel; and (3) an adhesive patch. Conductive gels reduce the impedance from the electrode to the skin. However, they dry up over time, which causes signal quality to deteriorate during long-term monitoring. Therefore, many materials have been explored as alternatives to traditional Ag/AgCl that are conformal to the skin. Since metals have high Young’s moduli, ultra-thin metal films can be arranged in serpentine or fractal geometries to provide flexibility or stretchability [17][18][19][20]. The stretchability of these thin-film metals can be increased by introducing conductive polymers. Polyethylene terephthalate (PET) and polydimethylsiloxane (PDMS) are commonly chosen as polymers due to their biocompatibility, wide availability, and low Young’s modulus [21]. In recent years, the conductive polymer poly (3,4-ethylene dioxthiophene): polystyrene trans acid (PEDOT:PSS) has been the most common polymer for textile-based electrodes due to its high sensitivity to biological molecules and high response time [22]. In addition to material advances, recent studies have decreased the impedance of surface electrodes by changing the form factor. For example, semi-invasive strategies, such as microneedle-based approaches, have been demonstrated to reduce motion artifacts [23][24].
3. Optoelectronic Signals
3.1. Mechanics of PPG
Photoplethysmography (PPG) is emerging as an alternative to ECG for cardiovascular monitoring due to its small size and ability to capture many different physiological parameters. The LED operates at red and near-infrared (NIR) frequencies, and the light intensity reaching the photodiode changes depending on the volumetric changes in the veins and arteries [25]. PPG sensors consist of two basic components: a light-emitting diode (LED) and a photodiode. The PPG sensor can function in two main modes: (1) transmission, where the LED and photodiode are placed on opposite sides of the medium; or (2) reflection, where the LED and photodiode are placed on the same side of the medium [26]. Due to the many factors affecting blood flow, including cardiac, neural, and respiratory factors, it is possible to look at many physiological parameters. PPG is currently used to measure several different aspects of heart health, including blood oxygen saturation (SpO2), blood pressure, heart rate, and respiratory rate [27][28][29][30][31].
3.2. Materials for Flexible PPG Devices
Both flexible LEDs and flexible diodes have been developed to reduce the effects of motion artifacts, which have been difficult to remove with filtering alone [32]. The most common photodiodes currently in use are silicon photodiodes, as they are widely available and flexible. Kim et al. used flexible (PIN) silicon diodes in combination with near-field communication (NFC) to deliver power, eliminating the need for a battery [33]. The photodiodes were paired with red and infrared LEDs, and the signals were amplified to coils and sent to a smartphone using the NFC platform. Li et al. offered an improvement on the conventional optoelectronic architecture by designing an epidermal silicon-based device by using a specific strain-isolation design, nanodiamond thinning, and hybrid transfer printing [34]. Gallium arsenide (GaAs) is a frequently used III-V semiconductor material that can be used as an alternative to Si-based materials based on its excellent charge-carrier mobility and high stability [35]. Organic materials for PPG sensors have become increasingly attractive due to their low fabrication cost and environmentally friendly footprint. For example, Yokota et al. developed a flexible pulse oximeter consisting of a polymer LED (PLED) and an organic photodiode (OPD) [36]. Exciting new optoelectronic research on quantum and nano-based materials is emerging thanks to the ability to tune the performance due to the size of the particles. Polat et al. introduced a photodiode made with graphene sensitized with semiconducting quantum dots [37]. Quantum-dot-based graphene photodetectors have high responsivity due to their built-in photoconductive gain.
4. Other Signals
4.1. Ultrasonic Signals
Doppler ultrasound has been used to track changes in arterial diameter, which can be used to track heart rate. However, commercially available ultrasound monitors are handheld and rigid and therefore not suitable for continuous monitoring. The active layer for ultrasonic transducers is most commonly lead zirconate titanate (PZT) or composites of PZT, as it exhibits high piezoelectric properties and high electromechanical properties [38].
4.2. Mechanoelectric Signals
Both ballistocardiography (BCG) and seismocardiography (SCG) have been used to measure cardiac activity based on the heart’s displacement, velocity, and accelerations. BCG measures entire body movement due to cardiac ejection, whereas SCG is a local chest measurement that registers cardiac-induced vibrations. Both are measured in terms of acceleration [39]. Since SCG is typically measured on the body, while BCG is measured using non-contact sensors, SCG has been more commonly used in wearable platforms. Ha et al. showed a stretchable e-tattoo SCG based on polyvinylidene fluoride (PVDF) [15]. Wearable phonocardiography (PCG) sensors have also been used to assess the heart. Accelerometer-based mechano-acoustic sensors function similarly to SCG and can pick up auditory frequencies that the human ear cannot hear with a traditional stethoscope. Flexible, wearable stethoscopes based on accelerometry have been developed with flexible substrates and electrodes [40][41].
4.3. Electrochemical Signals
Metabolic factors, such as glucose level, have been linked to increased risk for arrhythmias. However, monitoring of metabolite levels has traditionally been performed with invasive devices. Recently, wearable glucose-detection devices have allowed for repeated measurement of glucose levels without the burden of an implantable device [42]. Bandodkar et al. reported a tattoo-based noninvasive glucose-monitoring system based on Ag/AgCl ink electrodes and a reagent layer [43]. In vitro characterization proved the system’s ability to detect micromolar levels of glucose, and on-body evaluation proved its ability to detect a rise in glucose after a meal.
5. Arrhythmia Detection
Computer-aided ECG and PPG analysis has vastly improved the detection and classification of arrhythmias. The most current process for classification of arrhythmias consists of: (1) pre-processing, where baseline wander and unwanted noises and frequencies are filtered out; (2) feature extraction, where the most important features of a wave are identified, and (3) classification, where the most important features are input into a model to predict the class of arrhythmia of a given signal [44]. For filtering ECG signals, the P- and T-waves are typically found between 0.5 Hz and 10 Hz, while the QRS complex is found between 4 Hz and 20 Hz. Discrete wavelet transforms (DWTs) can be used in combination with low- and high-pass filters to remove unwanted frequencies [45][46]. For PPG signals, wavelet decomposition has also been investigated. However, filtering of motion artifacts for PPG signals has only been proven for weak noise, and very noisy PPG signals need to be discarded [47].
Machine learning models for both ECG and PPG signals have included support vector machine (SVM), multilayer perceptron (MLP), and decision tree (DT) models [48][49][50][51]. The cutting-edge machine learning area is deep learning. Deep learning methods, such as convolutional neural network (CNN), deep belief network (DBN), recurrent neural network (RNN), long short-term memory (LSTM), and gated recurrent unit (GRU), have been applied to arrhythmia detection [52][53][54][55][56].
6. Substrate Materials and Skin Interfaces
Due to their flexibility, ease of manufacturing, and low cost, substrates are often made from polymers or fabrics. Elastomers, such as PET, PI, PEN, polyetherimide (PEI), and parylene, are common materials for thin-film-based substrates. Their weak intramolecular forces enable greater elongation and therefore greater stretchability.
This entry is adapted from the peer-reviewed paper 10.3390/ma15030724
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2021 update. Circulation 2021, 143, e254–e743.
- Richards, K.J.C.; Cohen, A.T. Cardiac arrhythmias in the critically ill. Anaesth. Intensive Care Med. 2006, 7, 289–293.
- Kléber, A.G.; Rudy, Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004, 84, 431–488.
- Sardana, H.K.; Kanwade, R.; Tewary, S. Arrhythmia detection and classification using ECG and PPG techniques: A review. Phys. Eng. Sci. Med. 2021, 44, 1027–1048.
- Rho, R.W.; Page, R.L. Asymptomatic atrial fibrillation. Prog. Cardiovasc. Dis. 2005, 48, 79–87.
- Williams, S.E.; O’Neill, M.; Kotadia, I.D. Supraventricular tachycardia: An overview of diagnosis and management. Clin. Med. 2020, 20, 43.
- DiMarco, J.P.; Philbrick, J.T. Use of ambulatory electrocardiographic (Holter) monitoring. Ann. Intern. Med. 1990, 113, 53–68.
- Fung, E.; Järvelin, M.-R.; Doshi, R.N.; Shinbane, J.S.; Carlson, S.K.; Grazette, L.P.; Chang, P.M.; Sangha, R.S.; Huikuri, H.V.; Peters, N.S. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front. Physiol. 2015, 6, 149.
- Chen, Y.H.; Op de Beeck, M.; Vanderheyden, L.; Carrette, E.; Mihajlović, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; van Hoof, C. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors 2014, 14, 23758.
- Ackermans, P.A.J.; Solosko, T.A.; Spencer, E.C.; Gehman, S.E.; Nammi, K.; Engel, J.; Russell, J.K. A user-friendly integrated monitor-adhesive patch for long-term ambulatory electrocardiogram monitoring. J. Electrocardiol. 2012, 45, 148–153.
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69.
- Fayyaz Shahandashti, P.; Pourkheyrollah, H.; Jahanshahi, A.; Ghafoorifard, H. Highly conformable stretchable dry electrodes based on inexpensive flex substrate for long-term biopotential (EMG/ECG) monitoring. Sens. Actuators A Phys. 2019, 295, 678–686.
- Khan, Y.; Han, D.; Pierre, A.; Ting, J.; Wang, X.; Lochner, C.M.; Bovo, G.; Yaacobi-Gross, N.; Newsome, C.; Wilson, R.; et al. A flexible organic reflectance oximeter array. Proc. Natl. Acad. Sci. USA 2018, 115, E11015–E11024.
- Hamelmann, P.; Mischi, M.; Kolen, A.F.; van Laar, J.O.E.H.; Vullings, R.; Bergmans, J.W.M. Fetal heart-rate monitoring Implemented by dynamic adaptation of transmission power of a flexible ultrasound transducer array. Sensors 2019, 19, 1195.
- Ha, T.; Tran, J.; Liu, S.; Jang, H.; Jeong, H.; Mitbander, R.; Huh, H.; Qiu, Y.; Duong, J.; Wang, R.L.; et al. A chest-laminated ultrathin and stretchable e-tattoo for the measurement of electrocardiogram, seismocardiogram, and cardiac time intervals. Adv. Sci. 2019, 6, 1900290.
- Hammad, M.; Maher, A.; Wang, K.; Jiang, F.; Amrani, M. Detection of abnormal heart conditions based on characteristics of ECG signals. Measurement 2018, 125, 634–644.
- Dong, W.; Cheng, X.; Xiong, T.; Wang, X. Stretchable bio-potential electrode with self-similar serpentine structure for continuous, long-term, stable ECG recordings. Biomed. Microdevices 2019, 21, 6.
- Fan, J.A.; Yeo, W.H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 3266.
- Morikawa, Y.; Yamagiwa, S.; Sawahata, H.; Numano, R.; Koida, K.; Ishida, M.; Kawano, T.; Morikawa, Y.; Yamagiwa, S.; Sawahata, H.; et al. Ultrastretchable kirigami bioprobes. Adv. Healthc. Mater. 2018, 7, 1701100.
- Xu, B.; Akhtar, A.; Liu, Y.; Chen, H.; Yeo, W.-H.; Park, S.I.I.; Boyce, B.; Kim, H.; Yu, J.; Lai, H.-Y.; et al. An epidermal stimulation and sensing platform for sensorimotor prosthetic control, management of lower back exertion, and electrical muscle activation. Adv. Mater. 2016, 28, 4462–4471.
- Jung, H.; Moon, J.; Baek, D.; Lee, J.; Choi, Y.; Hong, J.; Lee, S. CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479.
- Pani, D.; Dessì, A.; Saenz-Cogollo, J.F.; Barabino, G.; Fraboni, B.; Bonfiglio, A. Fully textile, PEDOT:PSS based electrodes for wearable ECG monitoring systems. IEEE Trans. Biomed. Eng. 2016, 63, 540–549.
- Satti, A.T.; Park, J.; Park, J.; Kim, H.; Cho, S. Fabrication of parylene-coated microneedle array electrode for wearable ECG device. Sensors 2020, 20, 5183.
- Hou, Y.; Li, Z.; Wang, Z.; Yu, H. Miura-ori structured flexible microneedle array electrode for biosignal recording. Microsyst. Nanoeng. 2021, 7, 53.
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.H.; Abbott, D.; Lim, K.; Ward, R. The use of photoplethysmography for assessing hypertension. npj Digit. Med. 2019, 2, 60.
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable photoplethysmographic sensors—Past and present. Electronics 2014, 3, 282–302.
- Harfiya, L.N.; Chang, C.-C.; Li, Y.-H. Continuous blood pressure estimation using exclusively photopletysmography by LSTM-based signal-to-signal translation. Sensors 2021, 21, 2952.
- Lee, I.; Park, N.; Lee, H.; Hwang, C.; Kim, J.H.; Park, S. Systematic review on human skin-compatible wearable photoplethysmography sensors. Appl. Sci. 2021, 11, 2313.
- Askarian, B.; Jung, K.; Chong, J.W. Monitoring of heart rate from photoplethysmographic signals using a samsung galaxy note8 in underwater environments. Sensors 2019, 19, 2846.
- Mohan, P.M.; Nisha, A.A.; Nagarajan, V.; Jothi, E.S.J. Measurement of arterial oxygen saturation (SpO2) using PPG optical sensor. In Proceedings of the 2016 International Conference on Communication and Signal Processing (ICCSP), Melmaruvathur, India, 6–8 April 2016; pp. 1136–1140.
- L’Her, E.; N’Guyen, Q.-T.; Pateau, V.; Bodenes, L.; Lellouche, F. Photoplethysmographic determination of the respiratory rate in acutely ill patients: Validation of a new algorithm and implementation into a biomedical device. Ann. Intensive Care 2019, 9, 11.
- Chong, J.W.; Cho, C.H.; Tabei, F.; Le-Anh, D.; Esa, N.; Mcmanus, D.D.; Chon, K.H. Motion and noise artifact-resilient atrial fibrillation detection using a smartphone. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 230–239.
- Kim, J.; Salvatore, G.A.; Araki, H.; Chiarelli, A.M.; Xie, Z.; Banks, A.; Sheng, X.; Liu, Y.; Lee, J.W.; Jang, K.-I.; et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2022, 2, e1600418.
- Li, H.; Xu, Y.; Li, X.; Chen, Y.; Jiang, Y.; Zhang, C.; Lu, B.; Wang, J.; Ma, Y.; Chen, Y.; et al. Epidermal inorganic optoelectronics for blood oxygen measurement. Adv. Healthc. Mater. 2017, 6, 1601013.
- Hong, N.; Chu, R.J.; Kang, S.S.; Ryu, G.; Han, J.-H.; Yu, K.J.; Jung, D.; Choi, W.J. Flexible GaAs photodetector arrays hetero-epitaxially grown on GaP/Si for a low-cost III-V wearable photonics platform. Opt. Express 2020, 28, 36559–36567.
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856.
- Polat, E.O.; Mercier, G.; Nikitskiy, I.; Puma, E.; Galan, T.; Gupta, S.; Montagut, M.; Piqueras, J.J.; Bouwens, M.; Durduran, T.; et al. Flexible graphene photodetectors for wearable fitness monitoring. Sci. Adv. 2021, 5, eaaw7846.
- La, T.-G.; Le, L.H. Flexible and wearable ultrasound device for medical applications: A review on materials, structural designs, and current challenges. Adv. Mater. Technol. 2021, 2100798.
- Taebi, A.; Solar, B.E.; Bomar, A.J.; Sandler, R.H.; Mansy, H.A. Recent advances in seismocardiography. Vibration 2019, 2, 64–86.
- Liu, Y.; Norton, J.J.; Qazi, R.; Zou, Z.; Ammann, K.R.; Liu, H.; Yan, L.; Tran, P.L.; Jang, K.I.; Lee, J.W.; et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2021, 2, e1601185.
- Lee, K.; Ni, X.; Lee, J.Y.; Arafa, H.; Pe, D.J.; Xu, S.; Avila, R.; Irie, M.; Lee, J.H.; Easterlin, R.L.; et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 2020, 4, 148–158.
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, 1701150.
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2015, 87, 394–398.
- Hagiwara, Y.; Fujita, H.; Oh, S.L.; Tan, J.H.; Tan, R.S.; Ciaccio, E.J.; Acharya, U.R. Computer-aided diagnosis of atrial fibrillation based on ECG Signals: A review. Inf. Sci. 2018, 467, 99–114.
- Martis, R.J.; Acharya, U.R.; Adeli, H. Current methods in electrocardiogram characterization. Comput. Biol. Med. 2014, 48, 133–149.
- Addison, P.S. Wavelet transforms and the ECG: A review. Physiol. Meas. 2005, 26, R155–R199.
- Raghuram, M.; Madhav, K.V.; Krishna, E.H.; Komalla, N.R.; Sivani, K.; Reddy, K.A. Dual-tree complex wavelet transform for motion-artifact reduction of PPG signals. In Proceedings of the 2012 IEEE International Symposium on Medical Measurements and Applications Proceedings, Budapest, Hungary, 18–19 May 2012; pp. 1–4.
- Liu, S.; Shao, J.; Kong, T.; Malekian, R. ECG arrhythmia classification using high order spectrum and 2D graph fourier transform. Appl. Sci. 2020, 10, 4741.
- Shan, S.; Tang, S.; Huang, P.; Lin, Y.; Huang, W.; Lai, D.; Wu, A.A. Reliable PPG-based algorithm in atrial fibrillation detection. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, 17–19 October 2016; pp. 340–343.
- Kanawade, R.; Tewary, S.; Sardana, H.K. Photoplethysmography based arrhythmia detection and classification. In Proceedings of the 2019 6th International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 7–8 March 2019; pp. 944–948.
- Homaeinezhad, M.R.; Atyabi, S.A.; Tavakkoli, E.; Toosi, H.N.; Ghaffari, A.; Ebrahimpour, R. ECG arrhythmia recognition via a neuro-SVM–KNN hybrid classifier with virtual QRS image-based geometrical features. Expert Syst. Appl. 2012, 39, 2047–2058.
- Savalia, S.; Emamian, V. Cardiac arrhythmia classification by multi-layer perceptron and convolution neural networks. Bioengineering 2018, 5, 35.
- Singh, S.; Pandey, S.K.; Pawar, U.; Janghel, R.R. Classification of ECG arrhythmia using recurrent neural networks. Procedia Comput. Sci. 2018, 132, 1290–1297.
- Ebrahimi, Z.; Loni, M.; Daneshtalab, M.; Gharehbaghi, A. A review on deep learning methods for ECG arrhythmia classification. Expert Syst. Appl. X 2020, 7, 100033.
- Liu, M.; Kim, Y. Classification of heart diseases based on ecg signals using long short-term memory. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2707–2710.
- Sayantan, G.; Kien, P.T.; Kadambari, K.V. Classification of ECG beats using deep belief network and active learning. Med. Biol. Eng. Comput. 2018, 56, 1887–1898.
This entry is offline, you can click here to edit this entry!
