Biliary atresia is an aggressive liver disease of infancy and can cause death without timely surgical intervention. Early diagnosis of biliary atresia is critical to the recovery of bile drainage and long-term transplant-free survival. Ultrasound is recommended as the initial imaging strategy for the diagnosis of biliary atresia. Numerous ultrasound features have been proved helpful for the diagnosis of biliary atresia. In recent years, with the help of new technologies such as elastography ultrasound, contrast-enhanced ultrasound and artificial intelligence, the diagnostic performance of ultrasound has been significantly improved.
- biliary atresia
- imaging
- conventional ultrasound
- elastography
- percutaneous cholecystocholangiography
- artificial intelligence
1. Introduction
2. Conventional Ultrasound
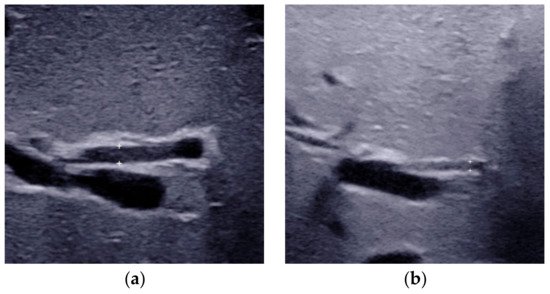
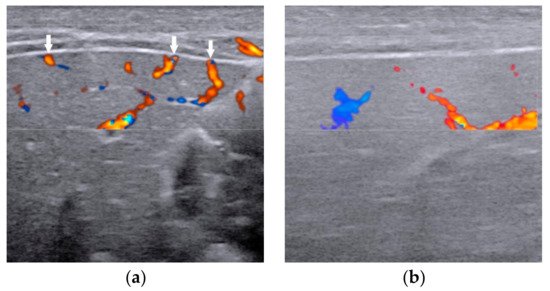
Conventional US is comprised of grey scale US and color Doppler US. With the help of constantly updated US equipment, the US features can be observed clearly better than ever before. High-frequency ultrasound (>10 MHz) is suggested for grey scale US and color Doppler to achieve the best spatial resolution. Numerous US features, especially the combination of gallbladder abnormalities and the triangular cord (TC) sign [6], have been proved helpful for diagnosing BA. Either of the two is positive, the infant should receive surgical exploration or cholangiography for the exclusion of BA.
2.1. Gallbladder Abnormalities
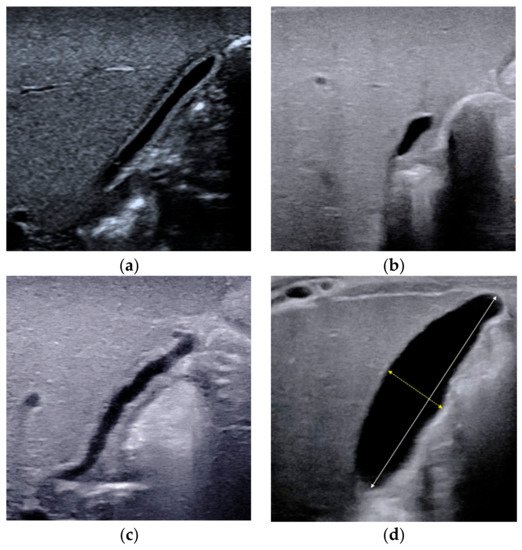
2.2. Triangular Cord Sign
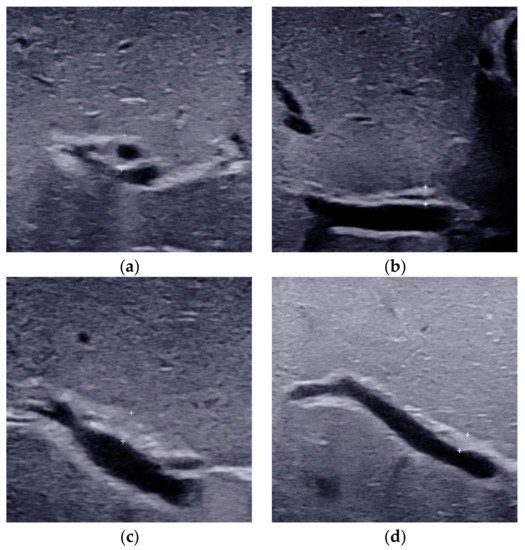
2.3. Porta Hepatis Macro- or Microcyst
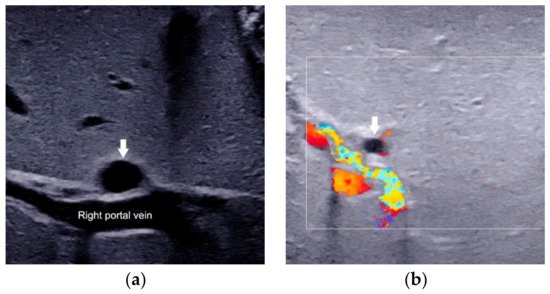
2.4. Enlarged Hepatic Hilar Lymph Node (LN)
2.5. Other Helpful US Features
3. Elastography
Elastography can be performed to quantify liver stiffness and fibrosis in infants with cholestasis [24][25][26] and to facilitate the differential diagnosis of BA [27][28][29][30][31][32][33][34][35][36][37]. With the development of elastography technology, various types of elastography have been reported for the diagnosis of BA.
Transient elastography (TE) is another type of quantitative elastography technology used to diagnose BA. A cut-off value of 7.7 kPa of TE yielded a sensitivity of 80% and specificity of 97% in infants younger than 90 days [32]. For the infants aged 91 to 180 days, a higher cutoff value of 8.8 kPa could yield higher diagnostic sensitivity (100%) and specificity (100%) [38]. However, the inability to choose different locations for the region of interest limits the clinical applicability of TE [39] in children.
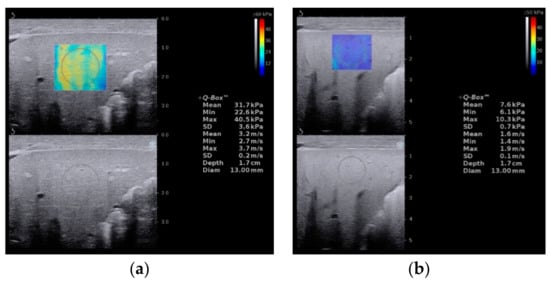
Supersonic shear wave elastography (SSWE) is a recently developed elastography system based on high frame-rate shear wave technology. It is based on capturing shear wave speed propagation, which presents a map of the elasticity in one area and allows stiffness quantitative analysis [27][28][34]. In our previous study, the cutoff value of SSWE for differentiating BA (Figure 7a) from non-BA (Figure 7b) was determined to be ≥10.2 kPa, with AUC 0.790, sensitivity 81.4% and specificity 66.7% [27].
4. US-Guided Percutaneous Cholecystocholangiography with Microbubbles
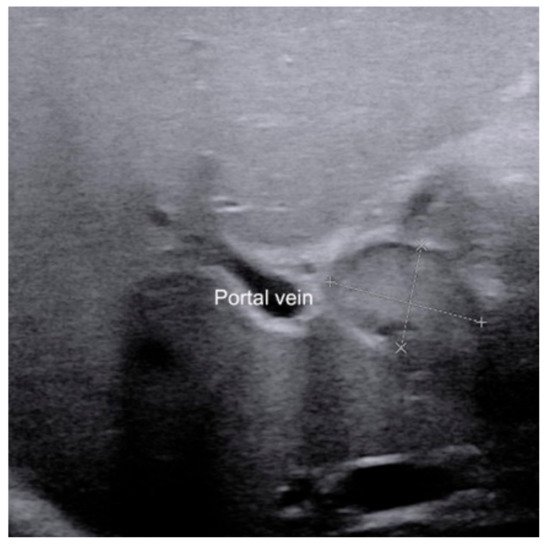
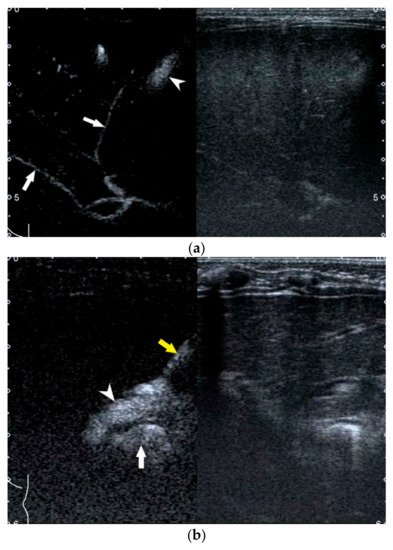
For those infants with a persistent rise of direct bilirubin level but equivocal US results, US-guided percutaneous cholecystocholangiography (PCC) may be used as another less invasive alternative to laparoscopic cholangiography if their gallbladder is full [40][41][42]. As previously reported, the diagnostic performance of US-guided PCC was better than that of conventional US [40][41]. Furthermore, it was helpful to be used for preoperatively differentiating subtypes of BA.
5. Artificial Intelligence Based on US Gallbladder Images
AI may have the potential to revolutionize BA diagnosis from US images particularly in rural area without relevant expertise.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12010051
References
- Chardot, C.; Carton, M.; Spire-Bendelac, N.; Le Pommelet, C.; Golmard, J.-L.; Auvert, B. Epidemiology of biliary atresia in France: A national study 1986–96. J. Hepatol. 1999, 31, 1006–1013.
- Hsiao, C.-H.; Chang, M.H.; Chen, H.-L.; Lee, H.-C.; Wu, T.-C.; Lin, C.-C.; Yang, Y.-J.; Chen, A.-C.; Tiao, M.-M.; Lau, B.-H.; et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology 2007, 47, 1233–1240.
- McKiernan, P.J.; Baker, A.J.; A Kelly, D. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet 2000, 355, 25–29.
- Hartley, J.L.; Davenport, M.; A Kelly, D. Biliary atresia. Lancet 2009, 374, 1704–1713.
- Shneider, B.L.; Brown, M.B.; Haber, B.; Whitington, P.F.; Schwarz, K.; Squires, R.; Bezerra, J.; Shepherd, R.; Rosenthal, P.; Hoofnagle, J.H.; et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J. Pediatr. 2006, 148, 467–474.e1.
- Zhou, L.; Shan, Q.; Tian, W.; Wang, Z.; Liang, J.; Xie, X. Ultrasound for the Diagnosis of Biliary Atresia: A Meta-Analysis. AJR Am. J. Roentgenol. 2016, 206, W73–W82.
- Farrant, P.; Meire, H.B.; Mieli-Vergani, G. Ultrasound features of the gall bladder in infants presenting with conjugated hyperbilirubinaemia. Br. J. Radiol. 2000, 73, 1154–1158.
- Humphrey, T.M.; Stringer, M.D. Biliary Atresia: US Diagnosis. Radiology 2007, 244, 845–851.
- Kendrick, A.P.A.T.; Phua, K.B.; Ooi, B.C.; Tan, C.E.L. Biliary atresia: Making the diagnosis by the gallbladder ghost triad. Pediatr. Radiol. 2003, 33, 311–315.
- Ikeda, S.; Sera, Y.; Akagi, M. Serial ultrasonic examination to differentiate biliary atresia from neonatal hepatitis—Special reference to changes in size of the gallbladder. Eur. J. Pediatrics 1989, 148, 396–400.
- McGahan, J.P.; E Phillips, H.; Cox, K.L. Sonography of the normal pediatric gallbladder and biliary tract. Radiology 1982, 144, 873–875.
- Zhou, L.-Y.; Wang, W.; Shan, Q.-Y.; Liu, B.-X.; Zheng, Y.-L.; Xu, Z.-F.; Xu, M.; Pan, F.-S.; Lu, M.-D.; Xie, X.-Y. Optimizing the US Diagnosis of Biliary Atresia with a Modified Triangular Cord Thickness and Gallbladder Classification. Radiology 2015, 277, 181–191.
- Lee, H.-J.; Lee, S.-M.; Park, W.-H.; Choi, S.-O. Objective Criteria of Triangular Cord Sign in Biliary Atresia on US Scans. Radiology 2003, 229, 395–400.
- Choi, S.-O.; Park, W.-H.; Lee, H.-J.; Woo, S.-K. ‘Triangular cord’: A sonographic finding applicable in the diagnosis of biliary atresia. J. Pediatr. Surg. 1996, 31, 363–366.
- Park, W.-H.; Choi, S.-O.; Lee, H.-J.; Kim, S.-P.; Zeon, S.-K.; Lee, S.-L. A new diagnostic approach to biliary atresia with emphasis on the ultrasonographic triangular cord sign: Comparison of ultrasonography, hepatobiliary scintigraphy, and liver needle biopsy in the evaluation of infantile cholestasis. J. Pediatr. Surg. 1997, 32, 1555–1559.
- Koob, M.; Pariente, D.; Habes, D.; Ducot, B.; Adamsbaum, C.; Franchi-Abella, S. The porta hepatis microcyst: An additional sonographic sign for the diagnosis of biliary atresia. Eur. Radiol. 2017, 27, 1812–1821.
- Caponcelli, E.; Knisely, A.S.; Davenport, M. Cystic biliary atresia: An etiologic and prognostic subgroup. J. Pediatr. Surg. 2008, 43, 1619–1624.
- Shan, Q.-Y.; Liu, B.-X.; Zhong, Z.-H.; Chen, H.-D.; Guo, Y.; Xie, X.-Y.; Zhou, W.-Y.; Jiang, H.; Zhou, L.-Y. The Prognosis of Type III Biliary Atresia with Hilar Cyst. Indian J. Pediatr. 2021, 88, 650–655.
- Weng, Z.; Zhou, L.; Wu, Q.; Zhou, W.; Ma, H.; Fang, Y.; Dang, T.; Liu, M. Enlarged hepatic hilar lymph node: An additional ultrasonographic feature that may be helpful in the diagnosis of biliary atresia. Eur. Radiol. 2019, 29, 6699–6707.
- Kim, W.S.; Cheon, J.-E.; Youn, B.J.; Yoo, S.-Y.; Kim, W.Y.; Kim, I.-O.; Yeon, K.M.; Seo, J.K.; Park, K.-W. Hepatic Arterial Diameter Measured with US: Adjunct for US Diagnosis of Biliary Atresia. Radiology 2007, 245, 549–555.
- El-Guindi, M.A.-S.; Sira, M.M.; Sira, A.M.; Salem, T.A.-H.; El-Abd, O.L.; Konsowa, H.A.-S.; El-Azab, D.S.; Allam, A.A.-H. Design and validation of a diagnostic score for biliary atresia. J. Hepatol. 2014, 61, 116–123.
- El-Guindi, M.A.-S.; Sira, M.M.; Konsowa, H.A.-S.; El-Abd, O.L.; Salem, T.A.-H. Value of hepatic subcapsular flow by color Doppler ultrasonography in the diagnosis of biliary atresia. J. Gastroenterol. Hepatol. 2013, 28, 867–872.
- Lee, M.S.; Kim, M.-J.; Lee, M.-J.; Yoon, C.S.; Han, S.J.; Oh, J.-T.; Park, Y.N. Biliary Atresia: Color Doppler US Findings in Neonates and Infants. Radiology 2009, 252, 282–289.
- Chen, H.; Zhou, L.; Liao, B.; Cao, Q.; Jiang, H.; Zhou, W.; Wang, G.; Xie, X. Two-Dimensional Shear Wave Elastography Predicts Liver Fibrosis in Jaundiced Infants with Suspected Biliary Atresia: A Prospective Study. Korean J. Radiol. 2021, 22, 959–969.
- Galina, P.; Alexopoulou, E.; Mentessidou, A.; Mirilas, P.; Zellos, A.; Lykopoulou, L.; Patereli, A.; Salpasaranis, K.; Kelekis, N.L.; Zarifi, M. Diagnostic accuracy of two-dimensional shear wave elastography in detecting hepatic fibrosis in children with autoimmune hepatitis, biliary atresia and other chronic liver diseases. Pediatr. Radiol. 2021, 51, 1358–1368.
- Gao, F.; Chen, Y.-Q.; Fang, J.; Gu, S.-L.; Li, L.; Wang, X.-Y. Acoustic Radiation Force Impulse Imaging for Assessing Liver Fibrosis Preoperatively in Infants with Biliary Atresia: Comparison with Liver Fibrosis Biopsy Pathology. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2017, 36, 1571–1578.
- Zhou, L.-Y.; Jiang, H.; Shan, Q.-Y.; Chen, D.; Lin, X.-N.; Liu, B.-X.; Xie, X.-Y. Liver stiffness measurements with supersonic shear wave elastography in the diagnosis of biliary atresia: A comparative study with grey-scale US. Eur. Radiol. 2017, 27, 3474–3484.
- Wang, X.; Qian, L.; Jia, L.; Bellah, R.; Wang, N.; Xin, Y.; Liu, Q. Utility of Shear Wave Elastography for Differentiating Biliary Atresia from Infantile Hepatitis Syndrome. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2016, 35, 1475–1479.
- Liu, Y.; Peng, C.; Wang, K.; Wu, D.; Yan, J.; Tu, W.; Chen, Y. The utility of shear wave elastography and serum biomarkers for diagnosing biliary atresia and predicting clinical outcomes. Eur. J. Pediatrics 2021, 30, 1–10.
- Sandberg, J.K.; Sun, Y.; Ju, Z.; Liu, S.; Jiang, J.; Koci, M.; Rosenberg, J.; Rubesova, E.; Barth, R.A. Ultrasound shear wave elastography: Does it add value to gray-scale ultrasound imaging in differentiating biliary atresia from other causes of neonatal jaundice? Pediatr. Radiol. 2021, 51, 1654–1666.
- Thumar, V.; Squires, J.H.; Spicer, P.J.; Robinson, A.L.; Chan, S.S. Ultrasound Elastography Applications in Pediatrics. Ultrasound Q. 2018, 34, 199–205.
- Wu, J.-F.; Lee, C.-S.; Lin, W.-H.; Jeng, Y.-M.; Chen, H.-L.; Ni, Y.-H.; Hsu, H.-Y.; Chang, M.-H. Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy. Hepatology 2018, 68, 616–624.
- Chen, S.; Liao, B.; Zhong, Z.; Zheng, Y.; Liu, B.; Shan, Q.; Xie, X.; Zhou, L. Supersonic shearwave elastography in the assessment of liver fibrosis for postoperative patients with biliary atresia. Sci. Rep. 2016, 6, 31057.
- Leschied, J.R.; Dillman, J.R.; Bilhartz, J.; Heider, A.; Smith, E.A.; Lopez, M.J. Shear wave elastography helps differentiate biliary atresia from other neonatal/infantile liver diseases. Pediatr. Radiol. 2015, 45, 366–375.
- Dillman, J.R.; DiPaola, F.W.; Smith, S.J.; Barth, R.A.; Asai, A.; Lam, S.; Campbell, K.M.; Bezerra, J.A.; Tiao, G.M.; Trout, A. Prospective Assessment of Ultrasound Shear Wave Elastography for Discriminating Biliary Atresia from other Causes of Neonatal Cholestasis. J. Pediatr. 2019, 212, 60–65.e3.
- Hanquinet, S.; Courvoisier, D.S.; Rougemont, A.-L.; Dhouib, A.; Rubbia-Brandt, L.; Wildhaber, B.E.; Merlini, L.; McLin, V.A.; Anooshiravani, M. Contribution of acoustic radiation force impulse (ARFI) elastography to the ultrasound diagnosis of biliary atresia. Pediatr. Radiol. 2015, 45, 1489–1495.
- Liu, Y.; Ni, X.; Pan, Y.; Luo, H. Comparison of the diagnostic value of virtual touch tissue quantification and virtual touch tissue imaging quantification in infants with biliary atresia. Int. J. Clin. Pr. 2021, 75, e13860.
- Boo, Y.; Chang, M.; Jeng, Y.; Peng, S.; Hsu, W.; Lin, W.; Chen, H.; Ni, Y.; Hsu, H.; Wu, J. Diagnostic Performance of Transient Elastography in Biliary Atresia among Infants with Cholestasis. Hepatol. Commun. 2021, 5, 882–890.
- Goldschmidt, I.; Streckenbach, C.; Dingemann, C.; Pfister, E.D.; di Nanni, A.; Zapf, A.; Baumann, U. Application and Limitations of Transient Liver Elastography in Children. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 109–113.
- Zhou, L.-Y.; Chen, S.-L.; Chen, H.-D.; Huang, Y.; Qiu, Y.-X.; Zhong, W.; Xie, X.-Y. Percutaneous US-guided Cholecystocholangiography with Microbubbles for Assessment of Infants with US Findings Equivocal for Biliary Atresia and Gallbladder Longer than 1.5 cm: A Pilot Study. Radiology 2018, 286, 1033–1039.
- Lee, S.Y.; Kim, G.C.; Choe, B.-H.; Ryeom, H.K.; Jang, Y.-J.; Kim, H.J.; Park, J.Y.; Cho, S.-M. Efficacy of US-guided Percutaneous Cholecystocholangiography for the Early Exclusion and Type Determination of Biliary Atresia. Radiology 2011, 261, 916–922.
- Treem, W.R.; Grant, E.E.; Barth, K.H.; Kramers, P.W. Ultrasound Guided Percutaneous Cholecystocholangiography for Early Differentiation of Cholestatic Liver Disease in Infants. J. Pediatr. Gastroenterol. Nutr. 1988, 7, 347–352.
- Zhou, W.; Yang, Y.; Yu, C.; Liu, J.; Duan, X.; Weng, Z.; Chen, D.; Liang, Q.; Fang, Q.; Zhou, J.; et al. Ensembled deep learning model outperforms human experts in diagnosing biliary atresia from sonographic gallbladder images. Nat. Commun. 2021, 12, 1259.
- Buda, M.; Wildman-Tobriner, B.; Hoang, J.K.; Thayer, D.; Tessler, F.N.; Middleton, W.D.; Mazurowski, M.A. Management of Thyroid Nodules Seen on US Images: Deep Learning May Match Performance of Radiologists. Radiology 2019, 292, 695–701.
