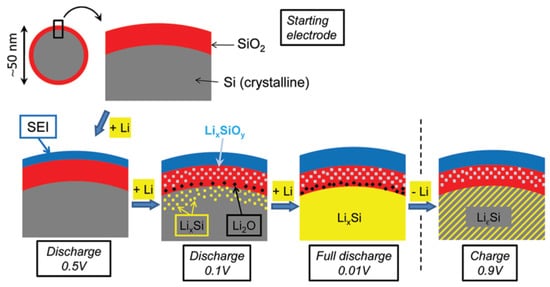
The structural and interfacial stability of silicon-based and lithium metal anode materials is essential to their battery performance. Scientists are looking for a better inactive material to buffer strong volume change and suppress unwanted surface reactions of these anodes during cycling. Lithium silicates formed in situ during the formation cycle of silicon monoxide anode not only manage anode swelling but also avoid undesired interfacial interactions, contributing to the successful commercialization of silicon monoxide anode materials. Additionally, lithium silicates have been further utilized in the design of advanced silicon and lithium metal anodes, and the results have shown significant promise in the past few years.
- silicon monoxide
- silicon
- metallic lithium
- anode
- energy storage
- protective layer
- volume expansion
1. Introduction
2. Structures of Lithium Silicates
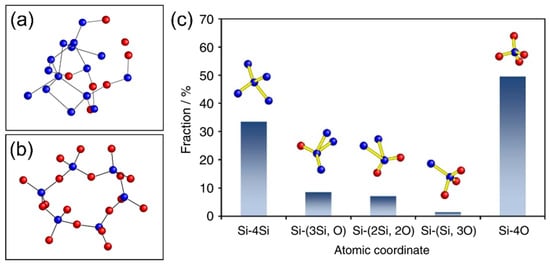
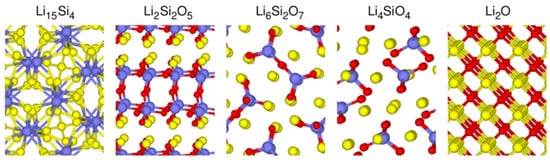
3. Electrochemical Properties and Formation Conditions of Lithium Silicates
3.1. Li4SiO4
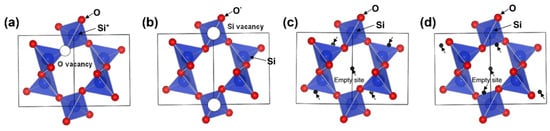
3.2. Li2SiO3
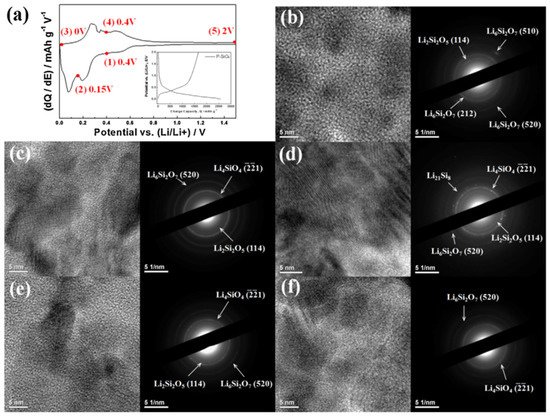
3.3. Li2Si2O5
3.4. Li6Si2O7
4. Lithium Silicates in Silicon, Lithium Metal, and Other Anode Materials
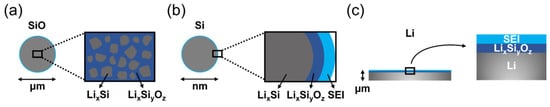
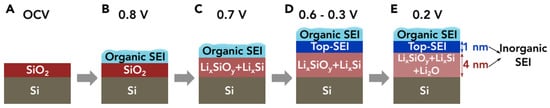
4.1. Lithium Silicates in Silicon Anodes
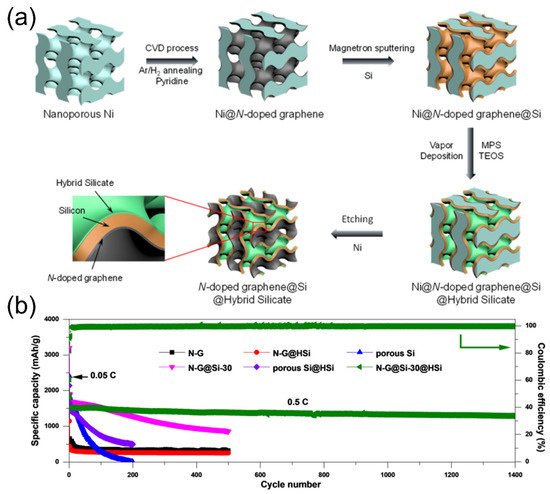
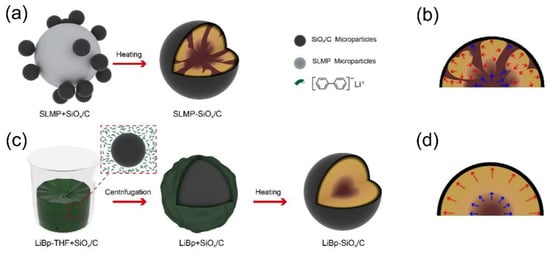
4.2. Lithium Silicates in Lithium Metal Anodes
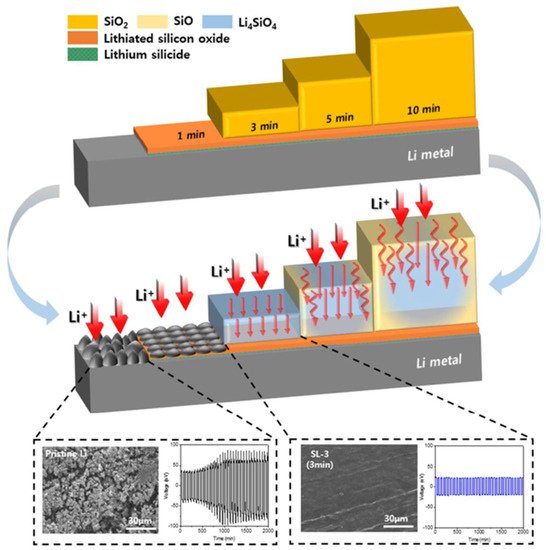
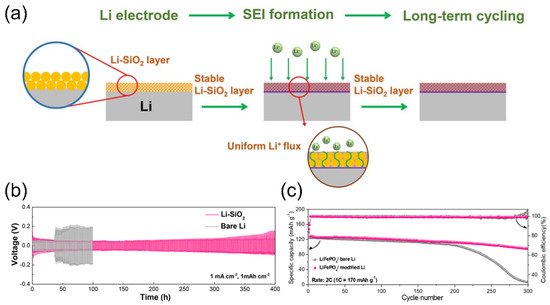
5. Summary and Outlook
This entry is adapted from the peer-reviewed paper 10.3390/batteries8010002
References
- Yamamura, H.; Nobuhara, K.; Nakanishi, S.; Iba, H.; Okada, S. Investigation of the Irreversible Reaction Mechanism and the Reactive Trigger on SiO Anode Material for Lithium-Ion Battery. J. Ceram. Soc. Jpn. 2011, 119, 855–860.
- Zhang, Y.; Guo, G.; Chen, C.; Jiao, Y.; Li, T.; Chen, X.; Yang, Y.; Yang, D.; Dong, A. An Affordable Manufacturing Method to Boost the Initial Coulombic Efficiency of Disproportionated SiO Lithium-Ion Battery Anodes. J. Power Sources 2019, 426, 116–123.
- Hohl, A.; Wieder, T.; van Aken, P.A.; Weirich, T.E.; Denninger, G.; Vidal, M.; Oswald, S.; Deneke, C.; Mayer, J.; Fuess, H. An Interface Clusters Mixture Model for the Structure of Amorphous Silicon Monoxide (SiO). J. Non-Cryst. Solids 2003, 320, 255–280.
- Kim, J.-H.; Park, C.-M.; Kim, H.; Kim, Y.-J.; Sohn, H.-J. Electrochemical Behavior of SiO Anode for Li Secondary Batteries. J. Electroanal. Chem. 2011, 661, 245–249.
- Chen, T.; Wu, J.; Zhang, Q.; Su, X. Recent Advancement of SiOx Based Anodes for Lithium-Ion Batteries. J. Power Sources 2017, 363, 126–144.
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531.
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715.
- Gu, M.; He, Y.; Zheng, J.; Wang, C. Nanoscale Silicon as Anode for Li-Ion Batteries: The Fundamentals, Promises, and Challenges. Nano Energy 2015, 17, 366–383.
- Pan, K.; Zou, F.; Canova, M.; Zhu, Y.; Kim, J.-H. Systematic Electrochemical Characterizations of Si and SiO Anodes for High-Capacity Li-Ion Batteries. J. Power Sources 2019, 413, 20–28.
- Hirata, A.; Kohara, S.; Asada, T.; Arao, M.; Yogi, C.; Imai, H.; Tan, Y.; Fujita, T.; Chen, M. Atomic-Scale Disproportionation in Amorphous Silicon Monoxide. Nat. Commun. 2016, 7, 11591.
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon Oxides: A Promising Family of Anode Materials for Lithium-Ion Batteries. Chem. Soc. Rev. 2019, 48, 285–309.
- Choi, I.; Lee, M.J.; Oh, S.M.; Kim, J.J. Fading Mechanisms of Carbon-Coated and Disproportionated Si/SiOx Negative Electrode (Si/SiOx/C) in Li-Ion Secondary Batteries: Dynamics and Component Analysis by TEM. Electrochim. Acta 2012, 85, 369–376.
- Jung, S.C.; Kim, H.-J.; Kim, J.-H.; Han, Y.-K. Atomic-Level Understanding toward a High-Capacity and High-Power Silicon Oxide (SiO) Material. J. Phys. Chem. C 2016, 120, 886–892.
- Sivonxay, E.; Aykol, M.; Persson, K.A. The Lithiation Process and Li Diffusion in Amorphous SiO2 and Si from First-Principles. Electrochim. Acta 2020, 331, 135344.
- Reynier, Y.; Vincens, C.; Leys, C.; Amestoy, B.; Mayousse, E.; Chavillon, B.; Blanc, L.; Gutel, E.; Porcher, W.; Hirose, T.; et al. Practical Implementation of Li Doped SiO in High Energy Density 21700 Cell. J. Power Sources 2020, 450, 227699.
- Cao, C.; Abate, I.I.; Sivonxay, E.; Shyam, B.; Jia, C.; Moritz, B.; Devereaux, T.P.; Persson, K.A.; Steinrück, H.-G.; Toney, M.F. Solid Electrolyte Interphase on Native Oxide-Terminated Silicon Anodes for Li-Ion Batteries. Joule 2019, 3, 762–781.
- Hirose, T.; Morishita, M.; Yoshitake, H.; Sakai, T. Study of Structural Changes That Occurred during Charge/Discharge of Carbon-Coated SiO Anode by Nuclear Magnetic Resonance. Solid State Ion. 2017, 303, 154–160.
- Liu, Y.; Wu, Y.; Zheng, J.; Wang, Y.; Ju, Z.; Lu, G.; Sheng, O.; Nai, J.; Liu, T.; Zhang, W.; et al. Silicious Nanowires Enabled Dendrites Suppression and Flame Retardancy for Advanced Lithium Metal Anodes. Nano Energy 2021, 82, 105723.
- Yu, B.-C.; Hwa, Y.; Kim, J.-H.; Sohn, H.-J. A New Approach to Synthesis of Porous SiOx Anode for Li-Ion Batteries via Chemical Etching of Si Crystallites. Electrochim. Acta 2014, 117, 426–430.
- Yu, B.-C.; Hwa, Y.; Park, C.-M.; Sohn, H.-J. Reaction Mechanism and Enhancement of Cyclability of SiO Anodes by Surface Etching with NaOH for Li-Ion Batteries. J. Mater. Chem. A 2013, 1, 4820.
- Hirose, T.; Morishita, M.; Yoshitake, H.; Sakai, T. Investigation of Carbon-Coated SiO Phase Changes during Charge/Discharge by X-Ray Absorption Fine Structure. Solid State Ion. 2017, 304, 1–6.
- Coyle, J.; Apblett, C.; Brumbach, M.; Ohlhausen, J.; Stoldt, C. Structural and Compositional Characterization of RF Magnetron Cosputtered Lithium Silicate Films: From Li2Si2O5 to Lithium-Rich Li8SiO6. J. Vac. Sci. Technol. A 2017, 35, 061509.
- Philippe, B.; Dedryvère, R.; Allouche, J.; Lindgren, F.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Nanosilicon Electrodes for Lithium-Ion Batteries: Interfacial Mechanisms Studied by Hard and Soft X-Ray Photoelectron Spectroscopy. Chem. Mater. 2012, 24, 1107–1115.
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A New Energy Storage Anode Material for Li-Ion Batteries. Energy Environ. Sci. 2012, 5, 6895.
- Veluchamy, A.; Doh, C.-H.; Kim, D.-H.; Lee, J.-H.; Lee, D.-J.; Ha, K.-H.; Shin, H.-M.; Jin, B.-S.; Kim, H.-S.; Moon, S.-I.; et al. Improvement of Cycle Behaviour of SiO/C Anode Composite by Thermochemically Generated Li4SiO4 Inert Phase for Lithium Batteries. J. Power Sources 2009, 188, 574–577.
- Doh, C.-H.; Veluchamy, A.; Oh, M.-W.; Han, B.-C. Analysis on the Formation of Li4SiO4 and Li2SiO3 through First Principle Calculations and Comparing with Experimental Data Related to Lithium Battery. J. Electrochem. Sci. Technol. 2011, 2, 146–151.
- Gong, Y.; Yu, X.; Yang, M.; Wei, J.; Shi, Y.; Huang, Z.; Lu, T.; Huang, W. A Facile Approach to Fabricate Li4SiO4 Ceramic Pebbles as Tritium Breeding Materials. Mater. Lett. 2015, 159, 245–248.
- Wang, K.; Yin, Z.; Zhao, P. Synthesis of Macroporous Li4SiO4 via a Citric Acid-Based Sol–Gel Route Coupled with Carbon Coating and Its CO2 Chemisorption Properties. Ceram. Int. 2016, 42, 2990–2999.
- Yang, S.; Wang, Q.; Miao, J.; Zhang, J.; Zhang, D.; Chen, Y.; Yang, H. Synthesis of Graphene Supported Li2SiO3 as a High Performance Anode Material for Lithium-Ion Batteries. Appl. Surf. Sci. 2018, 444, 522–529.
- Xiao, Y.; Wang, G.; Zhou, S.; Sun, Y.; Zhao, Q.; Gong, Y.; Lu, T.; Luo, C.; Yan, K. Enhanced Electrochemical Performance and Decreased Strain of Graphite Anode by Li2SiO3 and Li2CO3 Co-Modifying. Electrochim. Acta 2017, 223, 8–20.
- Wang, Q.; Yang, S.; Miao, J.; Lu, M.; Wen, T.; Sun, J. Graphene Supported Li2SiO3/Li4Ti5O12 Nanocomposites with Improved Electrochemical Performance as Anode Material for Lithium-Ion Batteries. Appl. Surf. Sci. 2017, 403, 635–644.
- Wang, Q.; Yang, S.; Miao, J.; Zhang, Y.; Zhang, D.; Chen, Y.; Li, Z. Synthesis of Graphene Supported Li2SiO3/Li2SnO3 Anode Material for Rechargeable Lithium Ion Batteries. Appl. Surf. Sci. 2019, 469, 253–261.
- Yom, J.H.; Hwang, S.W.; Cho, S.M.; Yoon, W.Y. Improvement of Irreversible Behavior of SiO Anodes for Lithium Ion Batteries by a Solid State Reaction at High Temperature. J. Power Sources 2016, 311, 159–166.
- Yan, M.-Y.; Li, G.; Zhang, J.; Tian, Y.-F.; Yin, Y.-X.; Zhang, C.-J.; Jiang, K.-C.; Xu, Q.; Li, H.-L.; Guo, Y.-G. Enabling SiOx/C Anode with High Initial Coulombic Efficiency through a Chemical Pre-Lithiation Strategy for High-Energy-Density Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 27202–27209.
- Alemi, A.; Khademinia, S.; Sertkol, M. Part III: Lithium Metasilicate (Li2SiO3)—Mild Condition Hydrothermal Synthesis, Characterization and Optical Properties. Int. Nano Lett. 2015, 5, 77–83.
- Wu, X.; Wen, Z.; Xu, X.; Wang, X.; Lin, J. Synthesis and Characterization of Li4SiO4 Nano-Powders by a Water-Based Sol-Gel Process. J. Nucl. Mater. 2009, 392, 471–475.
- Li, M.; Zeng, Y.; Ren, Y.; Zeng, C.; Gu, J.; Feng, X.; He, H. Fabrication and Lithium Storage Performance of Sugar Apple-Shaped SiOx@C Nanocomposite Spheres. J. Power Sources 2015, 288, 53–61.
- Sun, Q.; Zhang, B.; Fu, Z.-W. Lithium Electrochemistry of SiO2 Thin Film Electrode for Lithium-Ion Batteries. Appl. Surf. Sci. 2008, 254, 3774–3779.
- Lener, G.; Otero, M.; Barraco, D.E.; Leiva, E.P.M. Energetics of Silica Lithiation and Its Applications to Lithium Ion Batteries. Electrochim. Acta 2018, 259, 1053–1058.
- Alemi, A.; Khademinia, S.; Sertkol, M. Lithium Disilicate (Li2Si2O5): Mild Condition Hydrothermal Synthesis, Characterization and Optical Properties. J. Nanostruct. 2014, 4, 407–412.
- Koroleva, O.N.; Shtenberg, M.V.; Khvorov, P.V. Vibrational Spectroscopic and X-Ray Diffraction Study of Crystalline Phases in the Li2O-SiO2 System. Russ. J. Inorg. Chem. 2014, 59, 255–258.
- Wang, Y.T.; Cao, Y.D.; Hu, J.; Zhang, W.J.; Wu, D.P.; Shen, L. Fabrication of Lithium Silicate Doped with Lithium Titanate by Solid-State Reaction and Its XRD Study. AMR 2012, 624, 200–203.
- Prihandoko, B.; Sardjono, P.; Zulfia, A.; Siradj, E. The effect of Li2O on composite LTAP and windows glasses. Indones. J. Mater. Sci. 2007, 9, 6.
- Piazza, G.; Reimann, J.; Günther, E.; Knitter, R.; Roux, N.; Lulewicz, J.D. Behaviour of Ceramic Breeder Materials in Long Time Annealing Experiments. Fusion Eng. Des. 2001, 58–59, 653–659.
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882.
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon Based Lithium-Ion Battery Anodes: A Chronicle Perspective Review. Nano Energy 2017, 31, 113–143.
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473.
- Lin, D.; Liu, Y.; Cui, Y. Reviving the Lithium Metal Anode for High-Energy Batteries. Nat. Nanotech. 2017, 12, 194–206.
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213.
- Philippe, B.; Dedryvère, R.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Improved Performances of Nanosilicon Electrodes Using the Salt LiFSI: A Photoelectron Spectroscopy Study. J. Am. Chem. Soc. 2013, 135, 9829–9842.
- Schroder, K.W.; Celio, H.; Webb, L.J.; Stevenson, K.J. Examining Solid Electrolyte Interphase Formation on Crystalline Silicon Electrodes: Influence of Electrochemical Preparation and Ambient Exposure Conditions. J. Phys. Chem. C 2012, 116, 19737–19747.
- Xiong, J.; Yang, J.; Wang, G.; Saeed, T.; Liu, Y.; Kaczmarek, S.E.; Lu, W.; Wu, Q. Investigations on the Effect of Current Density on SiO/Si Composite Electrodes. Electrochim. Acta 2021, 393, 139072.
- Zhu, Y.; Hu, W.; Zhou, J.; Cai, W.; Lu, Y.; Liang, J.; Li, X.; Zhu, S.; Fu, Q.; Qian, Y. Prelithiated Surface Oxide Layer Enabled High-Performance Si Anode for Lithium Storage. ACS Appl. Mater. Interfaces 2019, 11, 18305–18312.
- Huang, G.; Han, J.; Lu, Z.; Wei, D.; Kashani, H.; Watanabe, K.; Chen, M. Ultrastable Silicon Anode by Three-Dimensional Nanoarchitecture Design. ACS Nano 2020, 14, 4374–4382.
- An, W.; Gao, B.; Mei, S.; Xiang, B.; Fu, J.; Wang, L.; Zhang, Q.; Chu, P.K.; Huo, K. Scalable Synthesis of Ant-Nest-like Bulk Porous Silicon for High-Performance Lithium-Ion Battery Anodes. Nat. Commun. 2019, 10, 1447.
- Ge, M.; Fang, X.; Rong, J.; Zhou, C. Review of Porous Silicon Preparation and Its Application for Lithium-Ion Battery Anodes. Nanotechnology 2013, 24, 422001.
- Ge, M.; Rong, J.; Fang, X.; Zhang, A.; Lu, Y.; Zhou, C. Scalable Preparation of Porous Silicon Nanoparticles and Their Application for Lithium-Ion Battery Anodes. Nano Res. 2013, 6, 174–181.
- Ge, M.; Rong, J.; Fang, X.; Zhou, C. Porous Doped Silicon Nanowires for Lithium Ion Battery Anode with Long Cycle Life. Nano Lett. 2012, 12, 2318–2323.
- Jia, H.; Zheng, J.; Song, J.; Luo, L.; Yi, R.; Estevez, L.; Zhao, W.; Patel, R.; Li, X.; Zhang, J.-G. A Novel Approach to Synthesize Micrometer-Sized Porous Silicon as a High Performance Anode for Lithium-Ion Batteries. Nano Energy 2018, 50, 589–597.
- Xiao, Q.; Gu, M.; Yang, H.; Li, B.; Zhang, C.; Liu, Y.; Liu, F.; Dai, F.; Yang, L.; Liu, Z.; et al. Inward Lithium-Ion Breathing of Hierarchically Porous Silicon Anodes. Nat. Commun. 2015, 6, 8844.
- Zhao, Y.; Liu, X.; Li, H.; Zhai, T.; Zhou, H. Hierarchical Micro/Nano Porous Silicon Li-Ion Battery Anodes. Chem. Commun. 2012, 48, 5079.
- Kim, J.Y.; Kim, A.-Y.; Liu, G.; Woo, J.-Y.; Kim, H.; Lee, J.K. Li4SiO4-Based Artificial Passivation Thin Film for Improving Interfacial Stability of Li Metal Anodes. ACS Appl. Mater. Interfaces 2018, 10, 8692–8701.
- Bieker, G.; Winter, M.; Bieker, P. Electrochemical in Situ Investigations of SEI and Dendrite Formation on the Lithium Metal Anode. Phys. Chem. Chem. Phys. 2015, 17, 8670–8679.
- Kang, H.-K.; Woo, S.-G.; Kim, J.-H.; Lee, S.-R.; Kim, Y.-J. Conductive Porous Carbon Film as a Lithium Metal Storage Medium. Electrochim. Acta 2015, 176, 172–178.
- Kuo, D.-H.; Yang, D.-G. Thick SiO2 Films Obtained by Plasma-Enhanced Chemical Vapor Deposition Using Hexamethyldisilazane, Carbon Dioxide, and Hydrogen. J. Electrochem. Soc. 2000, 147, 2679.
- Yuan, Y.; Wu, F.; Liu, Y.; Wang, X.; Zhang, K.; Zheng, L.; Wang, Z.; Bai, Y.; Wu, C. Rational Tuning of a Li4SiO4-Based Hybrid Interface with Unique Stepwise Prelithiation for Dendrite-Proof and High-Rate Lithium Anodes. ACS Appl. Mater. Interfaces 2020, 12, 39362–39371.
- Liu, F.; Xiao, Q.; Wu, H.B.; Shen, L.; Xu, D.; Cai, M.; Lu, Y. Fabrication of Hybrid Silicate Coatings by a Simple Vapor Deposition Method for Lithium Metal Anodes. Adv. Energy Mater. 2018, 8, 1701744.
- Yu, Y.; Yin, Y.-B.; Ma, J.-L.; Chang, Z.-W.; Sun, T.; Zhu, Y.-H.; Yang, X.-Y.; Liu, T.; Zhang, X.-B. Designing a Self-Healing Protective Film on a Lithium Metal Anode for Long-Cycle-Life Lithium-Oxygen Batteries. Energy Storage Mater. 2019, 18, 382–388.
- Ju, Z.; Jin, C.; Yuan, H.; Yang, T.; Sheng, O.; Liu, T.; Liu, Y.; Wang, Y.; Ma, F.; Zhang, W.; et al. A Fast-Ion Conducting Interface Enabled by Aluminum Silicate Fibers for Stable Li Metal Batteries. Chem. Eng. J. 2021, 408, 128016.
- Liang, W.; Lian, F.; Meng, N.; Lu, J.; Ma, L.; Zhao, C.-Z.; Zhang, Q. Adaptive Formed Dual-Phase Interface for Highly Durable Lithium Metal Anode in Lithium-Air Batteries. Energy Storage Mater. 2020, 28, 350–356.
- Stone, G.M.; Mullin, S.A.; Teran, A.A.; Hallinan, D.T.; Minor, A.M.; Hexemer, A.; Balsara, N.P. Resolution of the Modulus versus Adhesion Dilemma in Solid Polymer Electrolytes for Rechargeable Lithium Metal Batteries. J. Electrochem. Soc. 2012, 159, A222–A227.
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite Polymer Electrolytes for Lithium Batteries. Nature 1998, 394, 456–458.
- Rosero-Navarro, N.C.; Kajiura, R.; Jalem, R.; Tateyama, Y.; Miura, A.; Tadanaga, K. Significant Reduction in the Interfacial Resistance of Garnet-Type Solid Electrolyte and Lithium Metal by a Thick Amorphous Lithium Silicate Layer. ACS Appl. Energy Mater. 2020, 3, 5533–5541.
