The Ti/SnO2 interface has been investigated in situ via the technique of x-ray photoelectron spectroscopy. Thin films (in the range from 0.3 to 1.1 nm) of titanium were deposited on SnO2 substrates via the e-beam technique. The deposition was carried out at two different substrate temperatures, namely room temperature and 200 °C. The photoelectron spectra of tin and titanium in the samples were found to exhibit significant differences upon comparison with the corresponding elemental and the oxide spectra. These changes result from chemical interaction between SnO2 and the titanium overlayer at the interface. The SnO2 was observed to be reduced to elemental tin while the titanium overlayer was observed to become oxidized. Complete reduction of SnO2 to elemental tin did not occur even for the lowest thickness of the titanium overlayer. The interfaces in both the types of the samples were observed to consist of elemental Sn, SnO2, elemental titanium, TiO2, and Ti-suboxide.
- tin
- titanium
- tin oxide
- X-ray photoelectron spectroscopy
1. Introduction
2. Room Temperature Deposition
2.1. Sn 3d Region
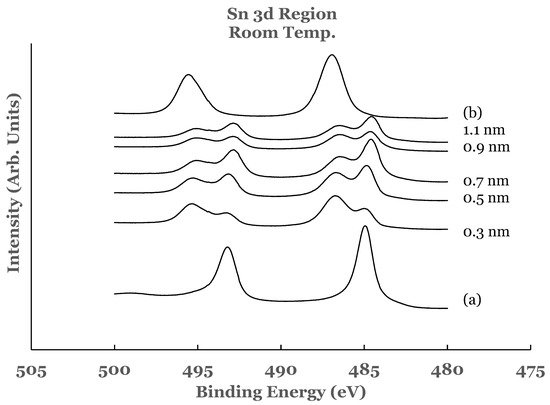
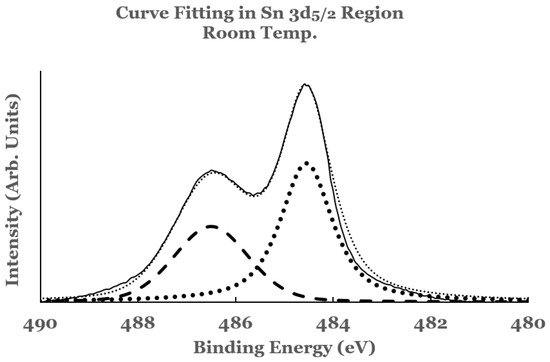
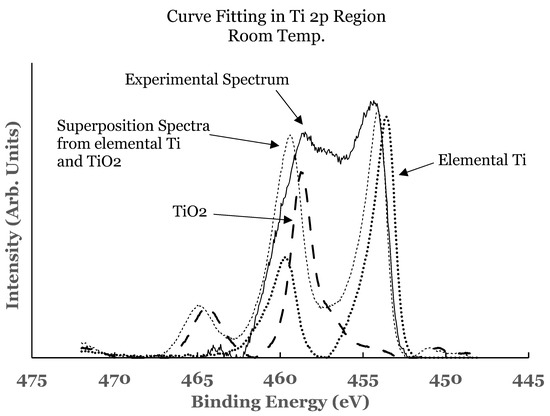
2.2. Ti 2p Region
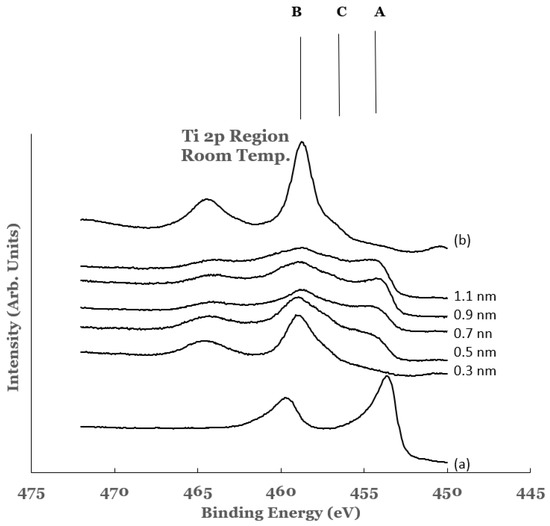
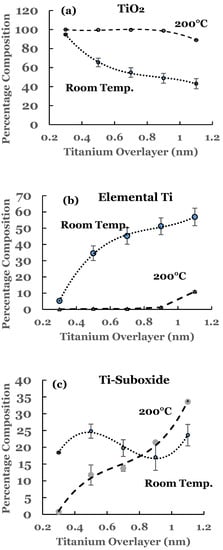
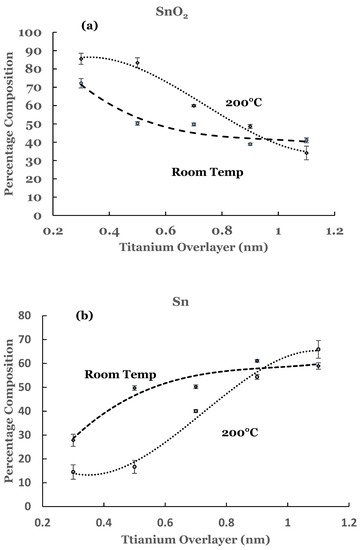
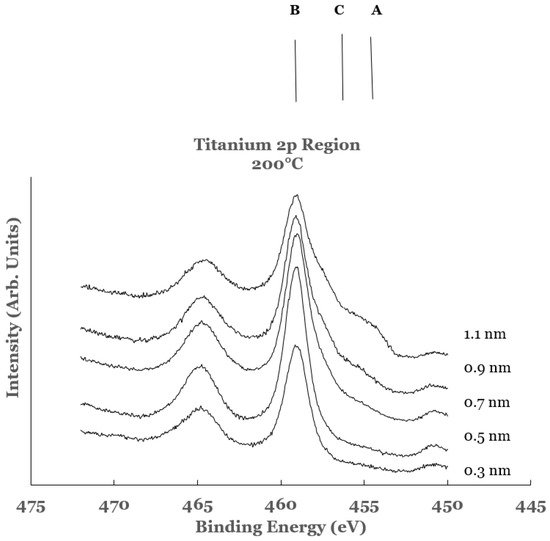
3. Substrate Temperature (200 °C) Deposition
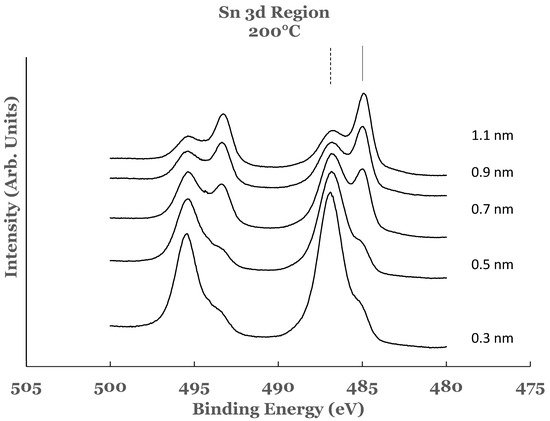
3.1. Sn 3d Region
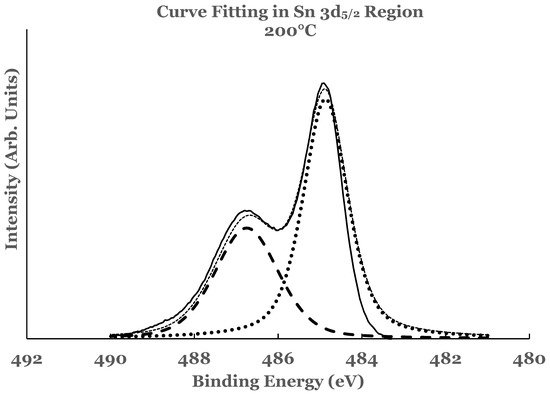
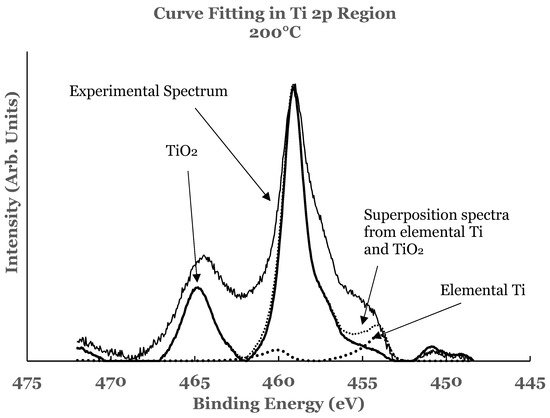
3.2. Ti 2p Region
This entry is adapted from the peer-reviewed paper 10.3390/nano12020202
References
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903.
- Liu, M.; Johnston, M.; Snaith, H. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398.
- Xiong, L.B.; Qin, M.C.; Chen, C.; Wen, J.; Yang, G.; Guo, Y.X.; Ma, J.J.; Zhang, Q.; Qin, P.L.; Li, S.Z.; et al. Fully High-Temperature-Processed SnO2 as Blocking Layer and Scaffold for Efficient, Stable, and Hysteresis-Free Mesoporous Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1706276.
- Park, M.; Kim, J.-Y.; Son, H.J.; Lee, C.-H.; Jang, S.S.; Ko, M.J. Low-temperature solution-processed Li-doped SnO2 as an effective electron transporting layer for high-performance flexible and wearable perovskite solar cells. Nano Energy 2016, 26, 208–215.
- Bai, Y.; Fang, Y.; Deng, Y.; Wang, Q.; Zhao, J.; Zheng, X.; Zhang, Y.; Huang, J. Low Temperature Solution-Processed Sb:SnO2 Nanocrystals for Efficient Planar Perovskite Solar Cells. ChemSusChem 2016, 9, 2686–2691.
- Chen, H.; Liu, D.; Wang, Y.; Wang, C.; Zhang, T.; Zhang, P.; Sarvari, H.; Chen, Z.; Li, S. Enhanced Performance of Planar Perovskite Solar Cells Using Low-Temperature Solution-Processed Al-Doped SnO2 as Electron Transport Layers. Nanoscale Res. Lett. 2017, 12, 1–6.
- Ren, X.; Yang, D.; Yang, Z.; Feng, J.; Zhu, X.; Niu, J.; Liu, Y.; Zhao, W.; Liu, S.F. Solution-Processed Nb:SnO2 Electron Transport Layer for Efficient Planar Perovskite Solar Cells. ACS Appl. Mater. Inter. 2017, 9, 2421–2429.
- Xiong, L.B.; Qin, M.C.; Yang, G.; Guo, Y.X.; Lei, H.W.; Liu, Q.; Ke, W.J.; Tao, H.; Qin, P.L.; Li, S.Z.; et al. Performance enhancement of high temperature SnO2-based planar perovskite solar cells: Electrical characterization and understanding of the mechanism. J. Mater. Chem. A 2016, 4, 8374–8383.
- Roose, B.; Johansen, C.M.; Dupraz, K.; Jaouen, T.; Aebi, P.; Steiner, U.; Abate, A. A Ga-doped SnO2 mesoporous contact for UV stable highly efficient perovskite solar cells. J. Mater. Chem. A 2017, 6, 1850–1857.
- Yahi, A.; Bouzidi, A.; Miloua, R.; Medles, M.; Nakrela, A.; Khadraoui, M.; Tabet-Derraz, H.; Desfeux, R.; Ferri, A.; Blach, J.-F. The relationship between processing and structural, optical, electrical properties of spray pyrolysed SnO2 thin films prepared for different deposition times. Optik 2019, 196.
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2016, 2, 16177.
- Barsan, N.; Schweizer-Berberich, M.; Göpel†, W. Fundamental and practical aspects in the design of nanoscaled SnO 2 gas sensors: A status report. Anal. Bioanal. Chem. 1999, 365, 287–304.
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106.
- Wang, B.; Zhu, L.F.; Yang, Y.H.; Xu, A.N.S.; Yang, G.W. Fabrication of a SnO2 Nanowire Gas Sensor and Sensor Performance for Hydrogen. J. Phys. Chem. C 2008, 112, 6643–6647.
- Nisar, J.; Topalian, Z.; De Sarkar, A.; Österlund, L.; Ahuja, R. TiO2-Based Gas Sensor: A Possible Application to SO2. ACS Appl. Mater. Interfaces 2013, 5, 8516–8522.
- Garzellaa, C.; Cominia, E.; Tempestia, E.; Frigerib, C.; Sberveglieri, G. TiO2 thin films by a novel sol–gel processing for gas sensor applications. Sens. Actuators 2000, B68, 189–196.
- Yu, J.; Zhao, X.; Zhao, Q. Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method. Thin Solid Films 2000, 379, 7–14.
- Yu, J.; Zhao, X.; Zhao, Q. Photocatalytic activity of nanometer TiO2 thin films prepared by the sol–gel method. Mater. Chem. Phys. 2001, 69, 25–29.
- Manjula, P.; Boppella, R.; Manorama, S.V. A Facile and Green Approach for the Controlled Synthesis of Porous SnO2 Nanospheres: Application as an Efficient Photocatalyst and an Excellent Gas Sensing Material. ACS Appl. Mater. Interfaces 2012, 4, 6252–6260.
- Liu, Y.; Jiao, Y.; Zhang, Z.; Qu, F.; Umar, A.; Wu, X. Hierarchical SnO2 Nanostructures Made of Intermingled Ultrathin Nanosheets for Environmental Remediation, Smart Gas Sensor, and Supercapacitor Applications. ACS Appl. Mater. Interfaces 2014, 6, 2174–2184.
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599.
- Wang, H.; Rogach, A.L. Hierarchical SnO2 Nanostructures: Recent Advances in Design, Synthesis, and Applications. Chem. Mater. 2013, 26, 123–133.
- Fortunato, E.; Ginley, D.; Hosono, H.; Paine, D.C. Transparent Conducting Oxides for Photovoltaics. MRS Bull. 2007, 32, 242–247.
- Green, A.N.M.; Palomares, D.C.; Haque, S.A.; Kroon, A.J.M.; Durrant, J.R. Charge Transport versus Recombination in Dye-Sensitized Solar Cells Employing Nanocrystalline TiO2 and SnO2 Films. J. Phys. Chem. B 2005, 109, 12525–12533.
- Lewis, B.G.; Paine, D.C. Applications and Processing of Transparent Conducting Oxides. MRS Bull. 2000, 25, 22–27.
- Dutta, S.; Patra, A.K.; De, S.; Bhaumik, A.; Saha, B. Self-Assembled TiO2 Nanospheres By Using a Biopolymer as a Template and Its Optoelectronic Application. ACS Appl. Mater. Interfaces 2012, 4, 1560–1564.
- Presley, R.E.; Munsee, C.L.; Park, C.-H.; Hong, D.; Wager, J.F.; Keszler, D. Tin oxide transparent thin-film transistors. J. Phys. D Appl. Phys. 2004, 37, 2810–2813.
- Bob, B.; Song, T.-B.; Chen, C.-C.; Xu, Z.; Yang, Y. Nanoscale Dispersions of Gelled SnO2: Material Properties and Device Applications. Chem. Mater. 2013, 25, 4725–4730.
- Vinodgopal, K.; Kamat, P.V. Enhanced rates of photocatalytic degradation of an azo dye using SnO2/TiO2 coupled semiconductor thin films. Environ. Sci. Technol. 1995, 29, 841–845.
- Liu, Z.; Sun, D.D.; Guo, P.; Leckie, J.O. An Efficient Bicomponent TiO2/SnO2 Nanofiber Photocatalyst Fabricated by Electrospinning with a Side-by-Side Dual Spinneret Method. Nano Lett. 2007, 7, 1081–1085.
- Beltrán, A.; Andrés, J.; Sambrano, J.R.; Longo, E. Density Functional Theory Study on the Structural and Electronic Properties of Low Index Rutile Surfaces for TiO2/SnO2/TiO2 and SnO2/TiO2/SnO2 Composite Systems. J. Phys. Chem. A 2008, 112, 8943–8952.
- Yadav, B.; Verma, N.; Singh, S. Nanocrystalline SnO2–TiO2 thin film deposited on base of equilateral prism as an opto-electronic humidity sensor. Opt. Laser Technol. 2012, 44, 1681–1688.
- Hou, X.; Wang, X.; Liu, B.; Wang, Q.; Wang, Z.; Chen, D.; Shen, G. SnO2@TiO2Heterojunction Nanostructures for Lithium-Ion Batteries and Self-Powered UV Photodetectors with Improved Performances. ChemElectroChem 2013, 1, 108–115.
- Radecka, M.; Zakrzewska, K.; Rerkas, M. SnO2–TiO2 solid solutions for gas sensors. Sens. Actuators B Chem. 1998, 47, 194–204.
- Chai, S.; Zhao, G.; Li, P.; Lei, Y.; Zhang, Y.; Li, D. Novel Sieve-Like SnO2/TiO2 Nanotubes with Integrated Photoelectrocatalysis: Fabrication and Application for Efficient Toxicity Elimination of Nitrophenol Wastewater. J. Phys. Chem. C 2011, 115, 18261–18269.
- Kumar, V.; Jaiswal, M.K.; Gupta, R.; Kulriya, P.K.; Asokan, K.; Sulania, I.; Ojha, S.; Kumar, R. Modification in the properties of SnO2 and TiO2 nanocomposite thin films by low energy ion irradiation. Integr. Ferroelectr. 2018, 193, 88–99.
- Huang, M.; Yu, J.; Li, B.; Deng, C.; Wang, L.; Wu, W.; Dong, L.; Zhang, F.; Fan, M. Intergrowth and coexistence effects of TiO2–SnO2 nanocomposite with excellent photocatalytic activity. J. Alloy. Compd. 2015, 629, 55–61.
- Vargas, M.A.; Rodríguez-Páez, J.E. Amorphous TiO2 nanoparticles: Synthesis and antibacterial capacity. J. Non-Crystalline Solids 2017, 459, 192–205.
- Wang, Q.; Wei, X.; Dai, J.; Jiang, J.; Huo, X. Influence of annealing process on ferromagnetism of undoped TiO2 nanoparticles prepared by sol–gel method. Mater. Sci. Semicond. Process. 2014, 21, 111–115.
- Kutuzova, A.S.; Dontsova, T.A. Characterization and properties of TiO2–SnO2 nanocomposites, obtained by hydrolysis method. Appl. Nanosci. 2019, 9, 873–880.
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Optical and structural characterization of TiO2 films doped with silver nanoparticles obtained by sol–gel method. Opt. Mater. 2013, 36, 207–213.
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51.
- Kovalev, I.A.; Petrov, A.A.; Ibragimova, O.A.; Shokod’ko, A.V.; Chernyavskii, A.S.; Goodilin, E.A.; Solntsev, K.A.; Tarasov, A.B. New hierarchical titania-based structures for photocatalysis. Mendeleev Commun. 2018, 28, 541–542.
- Rasoulnezhad, H.; Kavei, G.; Ahmadi, K.; Rahimipour, M.R. Combined sonochemical/CVD method for preparation of nanostructured carbon-doped TiO 2 thin film. Appl. Surf. Sci. 2017, 408, 1–10.
- Wittawat, R.; Rittipun, R.; Jarasfah, M.; Nattaporn, B. Synthesis of ZnO/TiO2 spherical particles for blue light screening by ultrasonic spray pyrolysis. Mater. Today Commun. 2020, 24, 101126.
- Bettini, L.; Dozzi, M.V.; Della Foglia, F.; Chiarello, G.L.; Selli, E.; Lenardi, C.; Piseri, P.; Milani, P. Mixed-phase nanocrystalline TiO2 photocatalysts produced by flame spray pyrolysis. Appl. Catal. B Environ. 2015, 178, 226–232.
- Scarisoreanu, M.; Fleaca, C.; Morjan, I.; Niculescu, A.-M.; Luculescu, C.; Dutu, E.; Ilie, A.; Morjan, I.; Florescu, L.G.; Vasile, E.; et al. High photoactive TiO 2 /SnO 2 nanocomposites prepared by laser pyrolysis. Appl. Surf. Sci. 2017, 418, 491–498.
- Anandan, S.; Pugazhenthiran, N.; Lana-Villarreal, T.; Lee, G.-J.; Wu, J.J. Catalytic degradation of a plasticizer, di-ethylhexyl phthalate, using Nx–TiO2−x nanoparticles synthesized via co-precipitation. Chem. Eng. J. 2013, 231, 182–189.
- Zikriya, M.; Nadaf, Y.; Bharathy, P.V.; Renuka, C. Luminescent characterization of rare earth Dy3+ ion doped TiO2 prepared by simple chemical co-precipitation method. J. Rare Earths 2018, 37, 24–31.
- Hariharan, D.; Thangamuniyandi, P.; Selvakumar, P.; Devan, U.; Pugazhendhi, A.; Vasantharaja, R.; Nehru, L. Green approach synthesis of 2 nanoparticles: Characterization, visible light active picric acid degradation and anticancer activity. Process Biochem. 2019, 87, 83–88.
- Papp, C.; Steinrück, H.-P. In situ high-resolution X-ray photoelectron spectroscopy—Fundamental insights in surface reactions. Surf. Sci. Rep. 2013, 68, 446–487.
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992.
- Cho, S.; Yu, J.; Kang, S.K.; Shih, D.-Y. Oxidation study of pure tin and its alloys via electrochemical reduction analysis. J. Electron. Mater. 2005, 34, 635–642.
- Thompson, A. X-ray Data Booklet; LBNL/PUB-490 Rev. 3; University of California: Berkeley, CA, USA, 2009.
- Kumar, D.; Chen, M.; Goodman, D. Characterization of ultra-thin TiO2 films grown on Mo(112). Thin Solid Films 2006, 515, 1475–1479.
- Dolat, D.; Mozia, S.; Wróbel, R.; Moszyński, D.; Ohtani, B.; Guskos, N.; Morawski, A.W. Nitrogen-doped, metal-modified rutile titanium dioxide as photocatalysts for water remediation. Appl. Catal. B Environ. 2015, 162, 310–318.
- Boscher, N.; Olivier, S.; Maurau, R.; Bulou, S.; Sindzingre, T.; Belmonte, T.; Choquet, P. Photocatalytic anatase titanium dioxide thin films deposition by an atmospheric pressure blown arc discharge. Appl. Surf. Sci. 2014, 311, 721–728.
- Saric, I.; Peter, R.; Petravic, M. Oxidation of Cobalt by Oxygen Bombardment at Room Temperature. J. Phys. Chem. C 2016, 120, 22421–22425.
- Dong, H.; Edmondson, J.; Miller, R.; Chourasia, A. Chemical reactivity at Fe/CuO interface studied in situ by X-ray photoelectron spectroscopy. Vacuum 2014, 101, 27–32.
- Gamsjager, H.; Gajda, T.; Sangster, J.; Saxena, S.K.; Voigt, W. Chemical Thermodynamics Volume 12; Perrone, J., Ed.; OECD Nuclear Energy Agency, Data Bank: Leoben, Austria, 2013; ISBN 978-92-64-99206-1.
- Schaefer, S.C. Electrochemical Determination of Thermodynamic Properties of Bismuth Sesquioxide and Stannic Oxide; United States Department of the Interior Report of Investigation 8906; Bureau of Mines Report of Investigations: Anchorage, AK, USA, 1984.
- Kim, J.; Kang, S. Stable phase domains of the TiO2–Ti3O5–Ti2O3–TiO–Ti(CxOy)–TiC system examined experimentally and via first principles calculations. J. Mater. Chem. A 2013, 2, 2641–2647.
- Lide, D.R. (Ed.) Handbook of Chemistry and Physics, 72nd ed.; CRC Press Inc.: Boca Raton, FL, USA, 1991.
- Magill, P.L. Equilibrium in the System Hydrogen, Water Vapor and the Oxides of Tin. Unpublished Bachelor’s Thesis, California Institute of Technology, Pasadena, CA, USA, 1923.
