Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
A sampling system that measures volatile particles needs appropriate design, dilution conditions and counting devices in order to capture the nucleation mode formation potential of the source (vehicle).
- primary aerosol
- fresh aerosol
- secondary aerosol
- nucleation mode
- vehicle emissions
- road transport
- urban pollution
- air quality
- PMP
- PEMS
1. Introduction
The measurement of volatile particles is a topic of debate, because they depend on many parameters. Small changes can result in orders of magnitude difference in the number concentration. The next section will summarize all these parameters and conclude with a proposal of a sampling system.
2. Sampling Conditions and (Semi)Volatile Particles
In the laboratory, the engine is connected to a dynamometer or the vehicle is placed on a chassis dynamometer. A prescribed “cycle” is followed where the engine speed and torque vary or, respectively, the vehicle speed profile. The whole exhaust gas is diluted in a dilution tunnel following constant volume sampling (CVS). The instruments sample diluted exhaust gas to determine the pollutants concentrations. For the determination of particulate matter (PM) mass emissions, a small portion of the diluted flow is extracted and passes through a filter. The filter mass change over the complete cycle determines the PM mass emissions. The temperature of the filter, depending on the regulatory context is maintained between 20 and 52 °C (EU light-duty) or 47 ± 5 °C (EU heavy-duty, USA). The actual dilution ratio varies over the test cycle since total diluted flow is kept constant: the dilution is high when the exhaust flow is low (idle and low speeds) and, vice versa, low when the exhaust flow is high (high speeds and loads). This is the opposite to what would be experienced with atmospheric dilution. Actually, both mixing and dilution ratio evolution until measurement differs between laboratory and atmospheric conditions. Furthermore, the dilution ratio evolution will be different for different vehicles, different CVS flow rates and different facilities. The influence of the dilution ratio on the PM mass has been discussed in the atmospheric science community [1], but also in the automotive emissions community [2][3][4]. It has been clearly shown that dilution ratio, temperature, and concentration of semi-volatile species all have significant influence on the partitioning of those species between the gas and particulate phases [5]. For example, decreasing the concentration of organics from 1 mg/m3 to 20 μg/m3 at 25 °C can cause approximately half of the condensed organics to evaporate [6].
PM increases at small dilution ratios (<5:1), as long as diluted exhaust temperature reaches ambient levels, due to the relatively high concentration of semi-volatile species that preferably partition to the particulate phase [1]. Compared to atmospheric dilution, the CVS exposes the exhaust at low dilution ratios for prolonged residence time before measurement, resulting in relatively high PM mass. Increasing the dilution reduces the PM mass as partial pressures of semi-volatile species drop, while diluted exhaust temperature is not significantly affected. This phenomenon is mostly visible with organic species. Inorganics such as sulfuric acid do not evaporate at typical ambient conditions once they have condensed on particles, because of their low volatility [6].
Even though the particulate mass of inorganics is rather insensitive to dilution ratio, this may not be the same for particle number. Actually, several studies have shown that the dilution air temperature, the dilution ratio, the relative humidity, and the residence time at such conditions affect nucleation mode particles [7][8][9][10][11]. The nucleation mode number concentration and mean size depend on the sampling conditions (dilution temperature, dilution ratio, relative humidity etc.) [9]. For the same dilution ratio, the lower the temperature the higher the nucleation mode [7][9][12]. Τhe trend was not so clear for the dilution ratio. Some studies found the maximum nucleation at the minimum dilution ratio they tested, e.g., 4:1 [10][13][14][15] or 12:1 [16][17][18][7][19][20]. However, another study found higher nucleation at 23:1 [9] than at dilution 9:1. A modeling study found the maximum nucleation at dilution ratio 15:1 due to the interaction of volatile precursors concentration, available soot surface and temperature [21]. Another exception was a study with humid air that found a higher nucleation mode with higher dilution ratios, probably due to the contribution of the humidity to the sulfuric acid growth [11]. Simplified calculations also estimate maximum nucleation at dilutions between 15–30:1 [22]. The residence (aging) time is also an important parameter because it results in bigger sizes due to particle growth from condensation of organics and agglomeration. Theoretical and experimental studies showed that the nucleation mode number concentration maximized at approximately 0.2 s after initial dilution, whereas particle diameter stabilized approximately 1 s later [23][24][25]. Other parameters might also be equally or even more important for the potential for nucleation mode formation, e.g., turbulence and the relative time constants for mixing and cooling [25][26][27], cooling of the tailpipe [28], presence of solid particles [18], length of sampling line, and storage/release of volatiles [22][29]. In general, the dilution corrected nucleation mode number concentration and mean size remain constant with dilutions >30:1 [13][20] or 50–60:1 [30]. Very high dilutions (range 200:1 to 1000:1) during chasing experiments did not reveal any changes of the size distributions [31]. Adding a secondary dilution to the primary dilution does not seem to change the characteristics of the nucleation mode [32].
The nucleation mode growth during the first seconds is very rapid (15 nm/s [23][24]). Similar rates are expected in the wake of the vehicle. Note the big difference compared to the growth of the nucleation mode in the atmosphere (diluted) (5 nm/h [33][34]). Such laboratory studies were conducted with high concentrations of sulfur and hydrocarbons, but newer studies with typical concentrations for modern vehicles are needed. Studies found that the organic species condensed onto the soot particles may contribute to 20–40% of the particle volume or mass for accumulation mode particles and up to 80% for nucleation mode particles [35]. Studies with diesel vehicles equipped with oxidation catalysts demonstrated that the condensed material contributed only to a few nm to the diameter of the accumulation mode [36]. The size changes of the accumulation mode due to condensation or agglomeration are typically small.
3. Laboratory vs. Real-Life Dilution
The agreement between laboratory and plume measurements for the accumulation mode is good in most studies [37][38][39][17]. The agreement in nucleation mode is not always as good [39][40]. Many studies have shown that the presence or absence of the “fresh” nucleation mode can be qualitatively reproduced in the laboratory [31]. However, the exact characteristics (concentration, mean size) depend on the conditions in both the atmosphere or in the laboratory. In most cases, the laboratory number concentrations were lower, probably due to the sampling conditions (relatively high dilution temperature, low relative humidity, lower turbulence in the diluters compared to the wake of the vehicle) [31][41]. However, nucleation mode mass may be higher in the laboratory due to the lower dilution and longer residence time [42]. One study showed that when ambient and laboratory conditions were matched the nucleation mode characteristics also matched [17]. However, to mimic the atmospheric dilution, a partial dilution system should, in principle, produce similar turbulent intensity. Since the ambient conditions are not constant and, even for the same vehicle, these would vary depending on the speed, mimicking any atmospheric process in the lab does not make sense. Instead, establishing sampling and dilution conditions that are preferable for nucleation mode formation would enable measuring the “nucleation formation potential” [43]. As discussed above, it is not clear whether this also could capture the secondary aerosol formation potential.
4. Desorption / Release
Nucleation mode particles have been observed at roadside measurements [44][45][46], chasing measurements [47], on-board the vehicle [48], and in the laboratory [42]. Thus, they are a “true” vehicle exhaust component, and not a measurement artifact. Nevertheless, storage and release of “condensable” material to/from the sampling lines, as sampling temperature varies may produce nucleation mode particles that are not part of the vehicle exhaust. Two cases will be discussed to clarify the existence of artifacts: (i) stored material at the lines and aftertreatment devices of the vehicle; and (ii) stored material at the transfer lines after the vehicle tailpipe when measuring these particles in a laboratory. In the second case the stored material might have been desorbed from previously tested vehicles and thus not related to the specific test under evaluation; the term particle “artifact” has also been used.
The storage and release influence on the particle number emissions has been assessed by many researchers measuring directly from the tailpipe or chasing vehicles. One study with a diesel vehicle without diesel particulate filter (DPF) reported that going from 50 km/h to 120 km/h resulted in a clear nucleation mode that its number concentration and mean size were decreasing over time [49]. Another study with a diesel vehicle (no DPF) showed that the nucleation mode at 100 and 120 km/h was different when ramping from a lower or higher speed [36]. Another older study with diesel engines (no DPF) showed that the nucleation mode depended on the exhaust gas temperature [13]. A few studies with diesel vehicles (no DPF) found that it takes some time (>25 min) to form the nucleation mode (e.g., at 100 km/h) [42][39]. It was suggested that one important parameter is the stored material at the catalyst and the exhaust gas temperature at the catalyst that determines the SO2 to SO3 conversion [42][50]. The previous studies were with vehicles utilizing high sulfur fuel (300 ppm). A study with a heavy-duty vehicle with a particulate filter and low sulfur fuel (15 ppm) also demonstrated the dependency of the nucleation mode on the exhaust gas temperature. A dedicated study with near zero sulfur fuel and low sulfur lubricant showed that the sulfur stored in the diesel oxidation catalyst (DOC) was the responsible for the nucleation mode formation [50].
In the laboratory environment, the transfer line that connects the vehicle tailpipe to the dilution tunnel inlet has also been shown to be a source of volatile and semi-volatile species that condense at times of decreasing temperature and are outgassed when the temperature rises [29]. This may lead to spontaneous nucleation in the sampling line, which is not a true vehicle exhaust component (particle “artifact”) [51][52]. In older studies with 0.7–1 m transfer lines and with older diesel engines without any aftertreatment it took >40 min until stabilization of the nucleation mode when the temperature increased from 200 °C to 300 °C [20][26]. In addition, the rate at which outgassing and resuspension take place is a function of the earlier operation history of the transfer line, which compromises the repeatability of the measurement [53]. This can affect PM mass measurements as well.
A study with a Euro 4 motorcycle found a huge nucleation mode particle number concentration during the high speed part of the cycle when measuring from the dilution tunnel. The nucleation mode was not formed (or evident) when some dilution took place at the tailpipe (i.e., open configuration). It was suggested that due to the lower temperatures at the transfer tube no desorption took place in the second case. In the first case, due to the high exhaust gas temperature, the desorbed material formed nucleation mode particles in the dilution tunnel (or pre-existing particles grew at the measurement range of the instrument, i.e., above 5 nm) [54]. Similarly, during DPF regeneration events a clear nucleation mode could be measured at the dilution tunnel, but not at the tailpipe (but with a different sampling system) [55].
The above mentioned phenomena refer to semi-volatile particles. For solid particles the effect is small. For a typical 6 m heated line, the solid particle number (SPN) emissions at the tailpipe and the dilution tunnel are similar within 15% [56]. Higher differences (35%) can be found during cold start where the concentrations are higher and agglomeration can result in lower concentrations at the dilution tunnel. However, for levels of non-DPF equipped vehicles (>1013 #/km) the effect can be significant: differences of >40% have been reported. The exact difference between tailpipe and dilution tunnel number concentrations depends on the initial levels and the lengths of the tube between the vehicle and the dilution tunnel [57].
To conclude, sampling from the tailpipe is necessary when total particles are being measured. This will not exclude storage and release phenomena, but these will be representative of the actual vehicle emissions and not sampling desorption artifacts.
Tailpipe measurements have some difficulties. One is that the exhaust flow measurement is needed, which introduces one more factor of uncertainty, along with proper time alignment of the different signals [58]. This is not a new issue for heavy-duty engines where this information is already available, but for light-duty vehicles the procedures (instruments, time alignment) need to be defined. One final difficulty is that sampling directly from the tailpipe means that the sampling system and probably the instruments should be in the same room with the vehicle, for sampling lines to be kept as short as possible. This could pose some difficulties when tests at low or high ambient temperatures are conducted. A study showed that such measurements could be conducted with the measurement instruments outside of the room, without influencing the results [59]. Systems for on board applications, e.g., portable emissions measurement systems (PEMS) are designed for such conditions, but the laboratory grade equipment are typically designed for typical laboratory conditions (e.g., 5–30 °C). The same applies to altitude simulation laboratories.
5. Instrumentation (Particle Counter)
Another point that needs to be addressed is the particle counter. In the European regulation for laboratory systems the counters are full flow condensation particle counters (CPCs). The reason is the high accuracy (i.e., no splitting inside the CPC) and the steep detection efficiency drop at the desired cut-point (low size limit with 50% detection efficiency) size. For 23 nm cut-point CPCs a strong effect of the particle material has been reported [60][61][62] on particle detectability, and similarly for 10 nm cut-point CPCs [63][64]. In general, smaller material dependency (in absolute numbers) is expected with higher saturator-condenser temperature difference [65]. Full flow CPCs with cut-points down to 4–5 nm are commercially available. A low cut-point size is important to capture the nucleation mode of modern vehicles, which might peak at small sizes. For example, one study with heavy-duty engines and low sulfur fuel and lubricant the nucleation mode was <10 nm, even though the residence time after the primary dilution was 3 s [66], also confirmed by theoretical calculations [67]. This was also found with CNG vehicles [68][69] or regenerating diesel vehicles [70].
Diffusion charging based systems are also used in PEMS as particle number counters. The reason is that diffusion chargers are robust for on-road use, without the need for working fluids [71], and less sensitive to ambient temperature differences. The size dependency is small with the new designs [72][73][74]. Permitting this concept in the regulation for laboratory systems would also allow real time size distribution measurements (which also include charging of particles). For example, the engine exhaust particle sizer (EEPS) [75], differential mobility spectrometer (DMS) [76], and electrical low pressure impactor (ELPI) [77][78] are commonly used in research [79]. It should be taken into account that their uncertainty is much higher than of CPCs, but in most cases it should remain at acceptable levels (30%) [79], in particular when solid particles are measured [80][81]. Measurements of volatile particles with size distribution measurement instruments can, however, have high differences in particle number concentrations (factor of 2) [81][82][83][84][85]. Particularities of each technology then should also be considered for regulatory measurements. For example, CPCs have a saturator temperature typically around 35–40 °C, thus making questionable the need for dilution at much lower temperatures. The ELPI has low pressure at the final stages for small particles, affecting the nucleation mode. ELPI measures based on aerodynamic equivalent diameter, while EEPS and DMS based on electrical mobility equivalent diameter [86]. When the density is not unified, the size classification is different at the two concepts. Such analysis is outside of the scope of this paper, but should be considered when deciding the appropriate instrumentation for regulatory purposes. Instruments measuring sub-10 nm particles have been reviewed elsewhere, and the differences are even higher [87].
Other instruments could also be used for research purposes, depending on the needs of the project. For example, an aerosol gas exchange system (AGES) could be used to separate volatiles for chemical characterization [88], or a filter holder to collect material on a filter for analysis.
6. Diluter and Particle Losses
The systems for regulatory purposes are characterized for varying losses according to particle size and these losses are taken into account in the so-called particle number concentration reduction factor (PCRF) in the 30–100 nm range. Penetration curves of various systems can be found in the literature [89]. In general, the agreement of systems corrected for the 30–100 nm losses is good, within 30% or better [90][91]. Such an approach does not cover the sub-30 nm range, where the losses are significant. Thus, size distributions peaking at sizes <30 nm can have big differences [58][92] when measured with different particle counters, even when correcting by the PCRF of each instrument.
Regarding volatile particles, the diluter can have an effect on the nucleation mode formation. For example, a study that compared a porous diluter at the tailpipe with the full dilution tunnel found big differences, even when the conditions were matched [15]. Other studies also found differences between different diluters (including ejector, porous, mini tunnel) [93], partly due to losses in the diluters [94] or heating one of the systems [30][95]. Better agreement was found when the diluters were heated and the nucleation mode was suppressed: ejector vs. rotating disk heated at 80 °C (no nucleation mode) [96]; mini tunnel vs. heated ejector [95]. In general, ejectors have been shown to have low particle losses in the nanometre range [32], but different designs can significantly affect the micrometre concentrations [97].
7. Recommended System
The recommended system is plotted in Figure 1. It is based on the conclusions of the Particulates project [43] and a review on measuring ultrafine particles [22], taking into account the current solid particle number (SPN) methodology [98]. Other approaches (e.g., using separate systems for solid and total particles) are also relevant alternatives. The key characteristics of the system are discussed below.
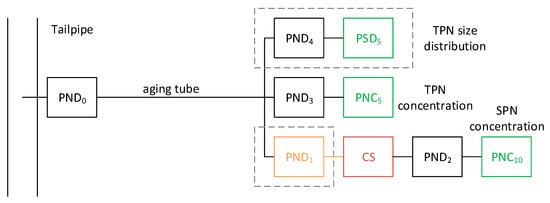
Figure 1. Recommended setup for measurements of solid and total particles. Red temperature > 300 °C. Green particle detectors. Dashed lines indicate optional parts. CS = catalytic stripper; PNC = particle number counter; PND = particle number diluter; PSD = particle size distribution instrument; SPN = solid particle number; TPN = total particle number.
The sampling line to the cold particle number diluter (PND0) should be as short as possible (residence time < 0.1 s) and heated at a temperature of >150 °C in order to avoid condensation, especially during cold starts. The dilution ratio should be around 12–25:1, and the dilution air temperature around 20–30 °C. The relative humidity of dilution air at the original setup was minimum (<5%). However, this should be re-considered as with the low sulphur fuels and lubricants humidity is important to grow the particles in the measurement range of the instruments and a higher value (e.g., around 50%) would be more representative of the ambient air. The diluted sample is then split in two paths.
One path is for the measurements of total particles. After an aging tube with residence time around 1–3 s to grow the nucleation mode within the lowest measurement size of the instruments (typically 5–10 nm), a second diluter follows (PND3), and then a particle number counter (PNC) with appropriate lower cut-point. Optionally, using a splitter, a particle size distribution instrument (PSD) can be used (with an appropriate diluter PND4). The recommended cut-point size of the instruments in this path is around 5 nm, possible with both commercial PSD and full flow PNCs [22]. Using a 10 nm PNC for total particles has the advantage of direct comparability with the solid path. Although sub-10 nm information will be lost, the calibration procedures are the ones already available for existing systems. It is important, however, to ensure that the nucleation mode (if any) has to grow to the 10 nm range.
The other path is the current Particle Measurement Programme (PMP) system for the measurement of solid particles. After a hot dilution (PND1) at 150 °C, a catalytic stripper (CS) removes the volatile particles [99]. A secondary dilution (PND2) is optional, but typically needed to reduce the particle concentration and reduce thermophoretic losses. The important is that the concentration is within the calibrated range of the particle counter (PNC) and the temperature of the sample below the maximum allowed temperature defined by the PNC. The PNC currently has a cut-point size of 23 nm. However, future regulations tend to lower the size to 10 nm.
The recommended system needs evaluation of PND0, but also of the particle losses especially at the 5–10 nm range, which in general is challenging. The expected reproducibility (but based on one system) was 22% [43][49] with non-DPF vehicles at the Particulates project. Higher values are expected for particulate filter equipped vehicles.
A simplified approach is to use PND1 of a PMP system as PND0 (i.e., without heating) and the current transfer lines as aging tube. Then, the path for the measurements of solid particles passes through the catalytic stripper, while the path for the measurement of total through the appropriate diluter and a PNC (5 nm or 10 nm). This approach will probably need higher dilutions at the PND2 than typically used in commercial systems, where the higher dilution is achieved with PND1.
References
- Shrivastava, M.K.; Lipsky, E.M.; Stanier, C.O.; Robinson, A.L. Modeling Semivolatile Organic Aerosol Mass Emissions from Combustion Systems. Environ. Sci. Technol. 2006, 40, 2671–2677.
- Macdonaid, J.S.; Plee, S.L.; D’Arcy, J.B.; Schreck, R.M. Experimental Measurements of the Independent Effects of Dilution Ratio and Filter Temperature on Diesel Exhaust Particulate Samples; SAE International: Warrendale, PA, USA, 1980.
- Durán, A. Accuracy of the European Standard Method to measure the amount of DPM emitted to the atmosphere. Fuel 2002, 81, 2053–2060.
- Vouitsis, E.; Ntziachristos, L.; Samaras, Z. Particulate matter mass measurements for low emitting diesel powered vehicles: What’s next? Prog. Energy Combust. Sci. 2003, 29, 635–672.
- Fujitani, Y.; Saitoh, K.; Fushimi, A.; Takahashi, K.; Hasegawa, S.; Tanabe, K.; Kobayashi, S.; Furuyama, A.; Hirano, S.; Takami, A. Effect of isothermal dilution on emission factors of organic carbon and n-alkanes in the particle and gas phases of diesel exhaust. Atmos. Environ. 2012, 59, 389–397.
- Robinson, A.L.; Grieshop, A.; Donahue, N.M.; Hunt, S. Updating the Conceptual Model for Fine Particle Mass Emissions from Combustion Systems Allen L. Robinson. J. Air Waste Manag. Assoc. 2010, 60, 1204–1222.
- Abdul-Khalek, I.; Kittelson, D.; Brear, F. The Influence of Dilution Conditions on Diesel Exhaust Particle Size Distribution Measurements; SAE International: Warrendale, PA, USA, 1999.
- Mathis, U.; Mohr, M.; Zenobi, R. Effect of organic compounds on nanoparticle formation in diluted diesel exhaust. Atmos. Chem. Phys. 2004, 4, 609–620.
- Mathis, U.; Ristimäki, J.; Mohr, M.; Keskinen, J.; Ntziachristos, L.; Samaras, Z.; Mikkanen, P. Sampling Conditions for the Measurement of Nucleation Mode Particles in the Exhaust of a Diesel Vehicle. Aerosol Sci. Technol. 2004, 38, 1149–1160.
- Lüders, H.; Krüger, M.; Stommel, P.; Lüers, B. The Role of Sampling Conditions in Particle Size Distribution Measurements; SAE International: Warrendale, PA, USA, 1998.
- Shi, J.P.; Harrison, R.M. Investigation of Ultrafine Particle Formation during Diesel Exhaust Dilution. Environ. Sci. Technol. 1999, 33, 3730–3736.
- Lucachick, G.; Curran, S.; Storey, J.; Prikhodko, V.; Northrop, W.F. Volatility characterization of nanoparticles from single and dual-fuel low temperature combustion in compression ignition engines. Aerosol Sci. Technol. 2016, 50, 436–447.
- Abdul-Khalek, I.S.; Kittelson, D.B.; Graskow, B.R.; Wei, Q.; Bear, F. Diesel Exhaust Particle Size: Measurement Issues and Trends; SAE International: Warrendale, PA, USA, 1998.
- Alozie, N.; Peirce, D.; Lindner, A.; Winklmayr, W.; Ganippa, L. Influence of Dilution Conditions on Diesel Exhaust Particle Measurement Using a Mixing Tube Diluter; SAE International: Warrendale, PA, USA, 2014.
- Louis, C.; Liu, Y.; Martinet, S.; D’Anna, B.; Valiente, A.M.; Boreave, A.; R’Mili, B.; Tassel, P.; Perret, P.; André, M. Dilution effects on ultrafine particle emissions from Euro 5 and Euro 6 diesel and gasoline vehicles. Atmos. Environ. 2017, 169, 80–88.
- Vaaraslahti, K.; Virtanen, A.; Ristimäki, J.; Keskinen, J. Nucleation Mode Formation in Heavy-Duty Diesel Exhaust with and without a Particulate Filter. Environ. Sci. Technol. 2004, 38, 4884–4890.
- Rönkkö, T.; Virtanen, A.; Lehtoranta, K.; Keskinen, J.; Pirjola, L.; Lappi, M. Effect of dilution conditions and driving parameters on nucleation mode particles in diesel exhaust: Laboratory and on-road study. Atmos. Environ. 2006, 40, 2893–2901.
- Vouitsis, E.; Ntziachristos, L.; Samaras, Z. Theoretical Investigation of the Nucleation Mode Formation Downstream of Diesel After-treatment Devices. Aerosol Air Qual. Res. 2008, 8, 37–53.
- Suresh, A.; Johnson, J.H. A Study of the Dilution Effects on Particle Size Measurement from a Heavy-Duty Diesel Engine with EGR; SAE International: Warrendale, PA, USA, 2001.
- DeSantes, J.M.; Bermúdez, V.; Pastor, J.V.; Fuentes, E. Methodology for measuring exhaust aerosol size distributions from heavy duty diesel engines by means of a scanning mobility particle sizer. Meas. Sci. Technol. 2004, 15, 2083–2098.
- Li, X.; Huang, Z. Formation and transformation of volatile nanoparticles from a diesel engine during exhaust dilution. Chin. Sci. Bull. 2012, 57, 948–954.
- Kittelson, D.; Khalek, I.; McDonald, J.; Stevens, J.; Giannelli, R. Particle emissions from mobile sources: Discussion of ultrafine particle emissions and definition. J. Aerosol Sci. 2021, 159, 105881.
- Vouitsis, E.; Ntziachristos, L.; Samaras, Z. Modelling of diesel exhaust aerosol during laboratory sampling. Atmos. Environ. 2005, 39, 1335–1345.
- Khalek, I.A.; Kittelson, D.B.; Brear, F. Nanoparticle Growth during Dilution and Cooling of Diesel Exhaust: Experimental Investigation and Theoretical Assessment; SAE International: Warrendale, PA, USA, 2000.
- Lemmetty, M.; Pirjola, L.; Mäkelä, J.M.; Rönkkö, T.; Keskinen, J. Computation of maximum rate of water–sulphuric acid nucleation in diesel exhaust. J. Aerosol Sci. 2006, 37, 1596–1604.
- Wei, Q.; Kittelson, D.B.; Watts, W.F. Single-Stage Dilution Tunnel Performance; SAE International: Warrendale, PA, USA, 2001.
- Singh, S.; Adams, P.J.; Misquitta, A.; Lee, K.J.; Lipsky, E.M.; Robinson, A.L. Computational Analysis of Particle Nucleation in Dilution Tunnels: Effects of Flow Configuration and Tunnel Geometry. Aerosol Sci. Technol. 2014, 48, 638–648.
- Ning, Z.; Cheung, C.S.; Liu, S. Experimental investigation of the effect of exhaust gas cooling on diesel particulate. J. Aerosol Sci. 2004, 35, 333–345.
- Yang, J.; Pham, L.; Johnson, K.C.; Durbin, T.D.; Karavalakis, G.; Kittelson, D.; Jung, H. Impacts of Exhaust Transfer System Contamination on Particulate Matter Measurements. Emiss. Control Sci. Technol. 2020, 6, 163–177.
- Lyyränen, J.; Jokiniemi, J.; Kauppinen, E.; Backman, U.; Vesala, H. Comparison of Different Dilution Methods for Measuring Diesel Particle Emissions. Aerosol Sci. Technol. 2004, 38, 12–23.
- Keskinen, J.; Rönkkö, T. Can Real-World Diesel Exhaust Particle Size Distribution be Reproduced in the Laboratory? A Critical Review Jorma Keskinen. J. Air Waste Manag. Assoc. 2010, 60, 1245–1255.
- Giechaskiel, B.; Ntziachristos, L.; Samaras, Z. Effect of ejector dilutors on measurements of automotive exhaust gas aerosol size distributions. Meas. Sci. Technol. 2009, 20, 045703.
- Hama, S.M.L.; Cordell, R.L.; Kos, G.P.A.; Weijers, E.P.; Monks, P.S. Sub-micron particle number size distribution characteristics at two urban locations in Leicester. Atmos. Res. 2017, 194, 1–16.
- Kerminen, V.-M.; Chen, X.; Vakkari, V.; Petäjä, T.; Kulmala, M.; Bianchi, F. Atmospheric new particle formation and growth: Review of field observations. Environ. Res. Lett. 2018, 13, 103003.
- Ristimäki, J.; Lehtoranta, K.; Lappi, M.; Keskinen, J. Hydrocarbon Condensation in Heavy-Duty Diesel Exhaust. Environ. Sci. Technol. 2007, 41, 6397–6402.
- Giechaskiel, B.; Chirico, R.; Decarlo, P.; Clairotte, M.; Adam, T.; Martini, G.; Heringa, M.; Richter, R.; Prevot, A.; Baltensperger, U.; et al. Evaluation of the particle measurement programme (PMP) protocol to remove the vehicles’ exhaust aerosol volatile phase. Sci. Total Environ. 2010, 408, 5106–5116.
- Casati, R.; Scheer, V.; Vogt, R.; Benter, T. Measurement of nucleation and soot mode particle emission from a diesel passenger car in real world and laboratory in situ dilution. Atmos. Environ. 2007, 41, 2125–2135.
- Giechaskiel, B.; Ntziachristos, L.; Samaras, Z.; Scheer, V.; Casati, R.; Vogt, R. Formation potential of vehicle exhaust nucleation mode particles on-road and in the laboratory. Atmos. Environ. 2005, 39, 3191–3198.
- Vogt, R.; Scheer, V.; Casati, R.; Benter, T. On-Road Measurement of Particle Emission in the Exhaust Plume of a Diesel Passenger Car. Environ. Sci. Technol. 2003, 37, 4070–4076.
- Sasaki, S.; Nakajima, T. Study on the Measuring Method of Vehicular PM Size Distribution to Simulate the Atmospheric Dilution Process; SAE International: Warrendale, PA, USA, 2002.
- Lee, S.H.; Kwak, J.H.; Lee, J.H. On-road chasing and laboratory measurements of exhaust particle emissions of diesel vehicles equipped with aftertreatment technologies (DPF, urea-SCR). Int. J. Automot. Technol. 2015, 16, 551–559.
- Giechaskiel, B.; Ntziachristos, L.; Samaras, Z.; Casati, R.; Scheer, V.; Vogt, R. Effect of Speed and Speed-Transition on the Formation of Nucleation Mode Particles from a Light Duty Diesel Vehicle; SAE International: Warrendale, PA, USA, 2007.
- Ntziachristos, L.; Samaras, Z. The Potential of a Partial-Flow Constant Dilution Ratio Sampling System as a Candidate for Vehicle Exhaust Aerosol Measurements. J. Air Waste Manag. Assoc. 2010, 60, 1223–1236.
- Ntziachristos, L.; Ning, Z.; Geller, M.D.; Sioutas, C. Particle Concentration and Characteristics near a Major Freeway with Heavy-Duty Diesel Traffic. Environ. Sci. Technol. 2007, 41, 2223–2230.
- Zimmerman, N.; Wang, J.M.; Jeong, C.-H.; Ramos, M.; Hilker, N.; Healy, R.M.; Sabaliauskas, K.; Wallace, J.S.; Evans, G.J. Field Measurements of Gasoline Direct Injection Emission Factors: Spatial and Seasonal Variability. Environ. Sci. Technol. 2016, 50, 2035–2043.
- Belkacem, I.; Helali, A.; Khardi, S.; Chrouda, A.; Slimi, K. Road traffic nanoparticle characteristics: Sustainable environment and mobility. Geosci. Front. 2021, 13, 101196.
- Kittelson, D.; Johnson, J.; Watts, W.; Wei, Q.; Drayton, M.; Paulsen, D.; Bukowiecki, N. Diesel Aerosol Sampling in the Atmosphere; SAE International: Warrendale, PA, USA, 2000.
- Bainschab, M.; Landl, L.; Andersson, J.; Mamakos, A.; Hausberger, S.; Bergmann, A. Measuring Sub-23 Nanometer Real Driving Particle Number Emissions Using the Portable DownToTen Sampling System. J. Vis. Exp. 2020, 159, e61287.
- Mamakos, A.; Ntziachristos, L.; Samaras, Z. Comparability of particle emission measurements between vehicle testing laboratories: A long way to go. Meas. Sci. Technol. 2004, 15, 1855–1866.
- Swanson, J.J.; Kittelson, D.B.; Watts, W.F.; Gladis, D.D.; Twigg, M.V. Influence of storage and release on particle emissions from new and used CRTs. Atmos. Environ. 2009, 43, 3998–4004.
- Maricq, M.M.; Chase, R.E.; Podsiadlik, D.H.; Vogt, R. Vehicle Exhaust Particle Size Distributions: A Comparison of Tailpipe and Dilution Tunnel Measurements; SAE International: Warrendale, PA, USA, 1999.
- Maricq, M.M.; Chase, R.E.; Xu, N. A comparison of tailpipe, dilution tunnel, and wind tunnel data in measuring motor vehicle PM. J. Air Waste Manag. Assoc. 2001, 51, 1529–1537.
- Maricq, M.M.; Szente, J.J.; Harwell, A.L.; Loos, M.J. Impact of aggressive drive cycles on motor vehicle exhaust PM emissions. J. Aerosol Sci. 2017, 113, 1–11.
- Giechaskiel, B. Gaseous and Particulate Emissions of a Euro 4 Motorcycle and Effect of Driving Style and Open or Closed Sampling Configuration. Sustainability 2020, 12, 9122.
- Giechaskiel, B. Particle Number Emissions of a Diesel Vehicle during and between Regeneration Events. Catalysts 2020, 10, 587.
- Giechaskiel, B.; Lähde, T.; Drossinos, Y. Regulating particle number measurements from the tailpipe of light-duty vehicles: The next step? Environ. Res. 2019, 172, 1–9.
- Isella, L.; Giechaskiel, B.; Drossinos, Y. Diesel-exhaust aerosol dynamics from the tailpipe to the dilution tunnel. J. Aerosol Sci. 2008, 39, 737–758.
- Giechaskiel, B.; Lähde, T.; Melas, A.D.; Valverde, V.; Clairotte, M. Uncertainty of laboratory and portable solid particle number systems for regulatory measurements of vehicle emissions. Environ. Res. 2021, 197, 111068.
- Ristimäki, J.; Keskinen, J.; Virtanen, A.; Maricq, M.; Aakko, P. Cold Temperature PM Emissions Measurement: Method Evaluation and Application to Light Duty Vehicles. Environ. Sci. Technol. 2005, 39, 9424–9430.
- Giechaskiel, B.; Wang, X.; Horn, H.-G.; Spielvogel, J.; Gerhart, C.; Southgate, J.; Jing, L.; Kasper, M.; Drossinos, Y.; Krasenbrink, A. Calibration of Condensation Particle Counters for Legislated Vehicle Number Emission Measurements. Aerosol Sci. Technol. 2009, 43, 1164–1173.
- Kiwull, B.; Wolf, J.-C.; Niessner, R. Response Characteristics of PMP Compliant Condensation Particle Counters Toward Various Calibration Aerosols. Aerosol Sci. Technol. 2015, 49, 98–108.
- Terres, A.; Giechaskiel, B.; Nowak, A.; Ebert, V. Calibration Uncertainty of 23nm Engine Exhaust Condensation Particle Counters with Soot Generators: A European Automotive Laboratory Comparison. Emiss. Control. Sci. Technol. 2021, 7, 124–136.
- Wang, X.; Caldow, R.; Sem, G.J.; Hama, N.; Sakurai, H. Evaluation of a condensation particle counter for vehicle emission measurement: Experimental procedure and effects of calibration aerosol material. J. Aerosol Sci. 2010, 41, 306–318.
- Yli-Ojanperä, J.; Sakurai, H.; Iida, K.; Mäkelä, J.M.; Ehara, K.; Keskinen, J. Comparison of Three Particle Number Concentration Calibration Standards Through Calibration of a Single CPC in a Wide Particle Size Range. Aerosol Sci. Technol. 2012, 46, 1163–1173.
- Giechaskiel, B.; Martini, G. Engine Exhaust Solid Sub-23 nm Particles: II. Feasibility Study for Particle Number Measurement Systems. SAE Int. J. Fuels Lubr. 2014, 7, 935–949.
- Vaaraslahti, K.; Keskinen, J.; Giechaskiel, B.; Solla, A.; Murtonen, T.; Vesala, H. Effect of Lubricant on the Formation of Heavy-Duty Diesel Exhaust Nanoparticles. Environ. Sci. Technol. 2005, 39, 8497–8504.
- Lemmetty, M.; Rönkkö, T.; Virtanen, A.; Keskinen, J.; Pirjola, L. The Effect of Sulphur in Diesel Exhaust Aerosol: Models Compared with Measurements. Aerosol Sci. Technol. 2008, 42, 916–929.
- Alanen, J.; Saukko, E.; Lehtoranta, K.; Murtonen, T.; Timonen, H.; Hillamo, R.; Karjalainen, P.; Kuuluvainen, H.; Harra, J.; Keskinen, J.; et al. The formation and physical properties of the particle emissions from a natural gas engine. Fuel 2015, 162, 155–161.
- Toumasatos, Z.; Kontses, A.; Doulgeris, S.; Samaras, Z.; Ntziachristos, L. Particle emissions measurements on CNG vehicles focusing on Sub-23 nm. Aerosol Sci. Technol. 2020, 55, 182–193.
- Transport & Environment. New Diesels, New Problems; European Federation for Transport and Environment AISBL: Brussels, Belgium, 2020.
- Fierz, M.; Houle, C.; Steigmeier, P.; Burtscher, H. Design, Calibration, and Field Performance of a Miniature Diffusion Size Classifier. Aerosol Sci. Technol. 2011, 45, 1–10.
- Giechaskiel, B.; Lähde, T.; Gandi, S.; Keller, S.; Kreutziger, P.; Mamakos, A. Assessment of 10-nm Particle Number (PN) Portable Emissions Measurement Systems (PEMS) for Future Regulations. Int. J. Environ. Res. Public Health 2020, 17, 3878.
- Schriefl, M.A.; Nishida, R.T.; Knoll, M.; Boies, A.M.; Bergmann, A. Characterization of particle number counters based on pulsed-mode diffusion charging. Aerosol Sci. Technol. 2020, 54, 772–789.
- Giechaskiel, B.; Mamakos, A.; Woodburn, J.; Szczotka, A.; Bielaczyc, P. Evaluation of a 10 nm Particle Number Portable Emissions Measurement System (PEMS). Sensors 2019, 19, 5531.
- Wang, X.; Grose, M.A.; Avenido, A.; Stolzenburg, M.R.; Caldow, R.; Osmondson, B.L.; Chow, J.C.; Watson, J. Improvement of Engine Exhaust Particle Sizer (EEPS) size distribution measurement—I. Algorithm and applications to compact-shape particles. J. Aerosol Sci. 2015, 92, 95–108.
- Biskos, G.; Reavell, K.; Collings, N. Description and Theoretical Analysis of a Differential Mobility Spectrometer. Aerosol Sci. Technol. 2005, 39, 527–541.
- Keskinen, J.; Pietarinen, K.; Lehtimäki, M. Electrical low pressure impactor. J. Aerosol Sci. 1992, 23, 353–360.
- Järvinen, A.; Aitomaa, M.; Rostedt, A.; Keskinen, J.; Yli-Ojanperä, J. Calibration of the new electrical low pressure impactor (ELPI+). J. Aerosol Sci. 2014, 69, 150–159.
- Giechaskiel, B.; Maricq, M.; Ntziachristos, L.; Dardiotis, C.; Wang, X.; Axmann, H.; Bergmann, A.; Schindler, W. Review of motor vehicle particulate emissions sampling and measurement: From smoke and filter mass to particle number. J. Aerosol Sci. 2014, 67, 48–86.
- Premnath, V.; Khalek, I.A.; Morgan, P. Relationship among Various Particle Characterization Metrics Using GDI Engine Based Light-Duty Vehicles; SAE International: Warrendale, PA, USA, 2018.
- Xue, J.; Li, Y.; Wang, X.; Durbin, T.D.; Johnson, K.C.; Karavalakis, G.; Asa-Awuku, A.; Villela, M.; Quiros, D.; Hu, S.; et al. Comparison of Vehicle Exhaust Particle Size Distributions Measured by SMPS and EEPS during Steady-State Conditions. Aerosol Sci. Technol. 2015, 49, 984–996.
- Rubino, L.; Phillips, P.R.; Twigg, M.V. Measurements of Ultrafine Particle Number Emissions from a Light-Duty Diesel Engine Using SMPS, DMS, ELPI and EEPS; SAE International: Warrendale, PA, USA, 2005.
- Price, P.; Stone, R.; Collier, T.; Davies, M.; Scheer, V. Dynamic Particulate Measurements from a DISI Vehicle: A Comparison of DMS500, ELPI, CPC and PASS; SAE International: Warrendale, PA, USA, 2006.
- Zervas, E.; Dorlhene, P. Comparison of Exhaust Particle Number Measured by EEPS, CPC, and ELPI. Aerosol Sci. Technol. 2006, 40, 977–984.
- Zerboni, A.; Rossi, T.; Bengalli, R.; Catelani, T.; Rizzi, C.; Priola, M.; Casadei, S.; Mantecca, P. Diesel exhaust particulate emissions and in vitro toxicity from Euro 3 and Euro 6 vehicles. Environ. Pollut. 2021, 297, 118767.
- Maricq, M.M.; Podsiadlik, D.H.; Chase, R.E. Size Distributions of Motor Vehicle Exhaust PM: A Comparison Between ELPI and SMPS Measurements. Aerosol Sci. Technol. 2000, 33, 239–260.
- Kangasluoma, J.; Cai, R.; Jiang, J.; Deng, C.; Stolzenburg, D.; Ahonen, L.R.; Chan, T.; Fu, Y.; Kim, C.; Laurila, T.M.; et al. Overview of measurements and current instrumentation for 1–10 nm aerosol particle number size distributions. J. Aerosol Sci. 2020, 148, 105584.
- Bainschab, M.; Martikainen, S.; Keskinen, J.; Bergmann, A.; Karjalainen, P. Aerosol gas exchange system (AGES) for nanoparticle sampling at elevated temperatures: Modeling and experimental characterization. Sci. Rep. 2019, 9, 17149.
- Giechaskiel, B.; Carriero, M.; Martini, G.; Krasenbrink, A.; Scheder, D. Calibration and Validation of Various Commercial Particle Number Measurement Systems. SAE Int. J. Fuels Lubr. 2009, 2, 512–530.
- Ntziachristos, L.; Giechaskiel, B.; Pistikopoulos, P.; Samaras, Z. Comparative Assessment of Two Different Sampling Systems for Particle Emission Type-Approval Measurements; SAE International: Warrendale, PA, USA, 2005.
- Giechaskiel, B.; Dilara, P.; Sandbach, E.; Andersson, J. Particle measurement programme (PMP) light-duty inter-laboratory exercise: Comparison of different particle number measurement systems. Meas. Sci. Technol. 2008, 19, 095401.
- Giechaskiel, B.; Woodburn, J.; Szczotka, A.; Bielaczyc, P. Particulate Matter (PM) Emissions of Euro 5 and Euro 6 Vehicles Using Systems with Evaporation Tube or Catalytic Stripper and 23 nm or 10 nm Counters; SAE International: Warrendale, PA, USA, 2020.
- Ntziachristos, L.; Saukko, E.; Lehtoranta, K.; Rönkkö, T.; Timonen, H.; Simonen, P.; Karjalainen, P.; Keskinen, J. Particle emissions characterization from a medium-speed marine diesel engine with two fuels at different sampling conditions. Fuel 2016, 186, 456–465.
- Dyakov, I.V.; Bergmans, B.; Idczak, F.; Blondeau, J.; Bram, S.; Cornette, J.; Coppieters, T.; Contino, F.; Mertens, J.; Breulet, H. Intercomparative measurements of particle emission from biomass pellet boiler with portable and stationary dilution devices. Aerosol Sci. Technol. 2021, 55, 665–680.
- Wong, C.; Chan, T.L.; Leung, C.W. Characterisation of diesel exhaust particle number and size distributions using mini-dilution tunnel and ejector–diluter measurement techniques. Atmos. Environ. 2003, 37, 4435–4446.
- Mohr, M.; Lehmann, U.; Margaria, G. ACEA Programme on the Emissions of Fine Particulates from Passenger Cars(2) Part 2: Effect of Sampling Conditions and Fuel Sulphur Content on the Particle Emission; SAE International: Warrendale, PA, USA, 2003.
- Shin, D.; Seo, H.; Hong, K.-J.; Kim, H.-J.; Kim, Y.-J.; Han, B.; Lee, G.-Y.; Chun, S.-N.; Hwang, J. Dilution Ratio and Particle Loss Performance of a Newly Developed Ejector Porous Tube Diluter Compared to a Commercial Diluter. Aerosol Air Qual. Res. 2020, 20, 2396–2403.
- Giechaskiel, B.; Mamakos, A.; Andersson, J.; Dilara, P.; Martini, G.; Schindler, W.; Bergmann, A. Measurement of Automotive Nonvolatile Particle Number Emissions within the European Legislative Framework: A Review. Aerosol Sci. Technol. 2012, 46, 719–749.
- Giechaskiel, B.; Melas, A.D.; Lähde, T.; Martini, G. Non-Volatile Particle Number Emission Measurements with Catalytic Strippers: A Review. Vehicles 2020, 2, 342–364.
This entry is offline, you can click here to edit this entry!
