Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
The study considers the phenolic hop compounds’ effect on the quality indicators of finished beer. The topic under consideration is relevant since it touches on the beer matrix colloidal stability when compounds with potential destabilizing activity are introduced into it from the outside.
- isoxanthogumol
- hop resins
- phenolic compounds
- β-glucan
- soluble nitrogen content
- taste beer descriptors
- dry hopping
1. Introduction
The beer’s quality as a colloidal system is determined by the organic compounds that form its structure. The grain (malted and unmalted cultures), the plant raw material (hops and hop products), and the yeast strain ultimately determine the sensory profile of the finished beer [1]. It is confirmed that, on the one hand, the overall complexity of the beer proteome is narrow and is caused by the protein molecules of Hordeum vulgare (barley) as a primary source, and yeast, but, on the other hand, there is a large variety of post-translational modifications (PTMs) created as a result of hydrolytic cleavage (proteolysis), glycation, and glycosylation during malting, mashing, and other technological stages of brewing. In particular, it is the protein molecules bound to non-protein components by covalent, ionic, van der Waals interactions that determine the structure and properties of beer [1,2].
Polyphenolic compounds from both grain raw materials and hops are important, in terms of functionality, in relation to beer quality.
Of all the classes of phenolic compounds, grains (rice, barley, wheat, and oats) are rich in phenolic acids, flavonoids, lignans, and tocols [3].
Hops contain phenolic acids, flavan-3-ols, and flavanols, including condensed, such as cereals, and authentic prenylflavanoids (xanthogumol, 6-,8-prenylnaringenin) and stilbenes in their composition [4].
It is reported that the malt (by 70 ÷ 80%) and the hops (by 20 ÷ 30%) contribute to the phenolic profile of the beer [5]. The profile consists of simple phenols; benzoic and cinnamic acid derivatives; coumarins; catechins; di-, tri-, and oligomeric proanthocyanidins; (prenylated) chalcones and flavonoids; as well as alpha- and iso-alpha acids [5]. Their wide range of variations determines the type of intermolecular interactions with protein molecules and the direction of their influence.
The polyphenols of both malt and hop under conditions of kettle hopping were found to optimize the reducing activity and reduce the carbonyl content during the fermentation process; they reduced, in particular, the intensity of the “harsh taste” in fresh beer, and in general, had a positive effect on flavor stability. However, plant polyphenols have been shown to have a negative effect on haze stability [6].
The hop’s phenolic compounds are still under study to this day. It has been shown that hops (Humulus lupulus L.) contain several physiologically active polyphenols important both for brewing and for other industries due to their potential, which is determined by genetic factors [7].
Various phenolic compounds, including hops, transferred into the beer affect the taste in general and, in particular, its taste fullness, and cause astringency and colloidal formation: flavonoid oxidation affect astringency, haze, and color, and low-molecular phenols (4-vinylsyringol) can give the beer extraneous aromas during storage (Table 1) [8].
Table 1. Phenol profile of beer.
| Phenol Class/Compound | Associate Compounds | Plant Issue | Teste Contribution | References |
|---|---|---|---|---|
| Catechins (flavan-3-ols) | bitterness harsh, medicinal, and metallic |
[9,10,11,12,13] | ||
| (+)-catechin | not associated | cereal/hop | ||
| (−)-epicatechin | not associated | hop | ||
| (+)-catechin | gallic acid | cereal | ||
| (−)-epicatechin | gallic acid, 4′-O-Methyl, glucuronic acid | hop | ||
| Proanthocyanidins (condensation products of flavan-3-ol monomers) Procianidins (di-, tri-, and tetra-catechin and epicatechin associated monomers) |
gallic acid, 4′-O-Methyl, glucuronic acid | hop | bitterness | [9,10,11,12,13] |
| Prodelfinidins (gallocatec hin, epigallocatechin, and di-, tri-, and tetramers) |
cereal | |||
| Flavanones Isoxanthohumol 6- and 8-prenylnaringenin 6-geranylnarin-genin |
residual glucose | hop | bitterness | [14] |
| Flavones apigenin chrysoeriol tricin |
residual glucose | cereals | astringency | [15] |
| Flavonols kaempferol quercetin rutin |
residual glucose | cereals hop |
bitterness | [16] |
| Monophenols Gallic acid, protocatechic acid, caffeic acid, vanillic acid, ferulic acid, p-coumaric acid, syringic acid, and their aldehydes |
bound form as esters, glycosides, and bound complexes | cereal hop |
harsh, bitter–sweet, sour, astringent, peppery, medicinal woody, roasted |
[17,18,19,20] |
In addition, flavonoids (taxifolin-o-glucoside, quercetin-o-glucoside, apigenin-6,8-dipentoside, and isofraxidin-o-glucoside) and phenolamides were also confirmed in beer [9].
There is a difference between phenolic compounds of malt and hops: despite the small number of phenolic compounds released into wort and beer compared to malt, hop polyphenols are more reactive with respect to protein deposition, which is associated with polarity and the polymerization index (degree of oxidation); in other words, the lower the polymerization index of polyphenols, the more active they are in association with wort proteins [21]. The polarity of phenolic compounds depends directly on the degree of electron cloud coverage of the atoms inside the molecules [22]. Moreover, the phenolic molecule associated with another compound already has a lower polarity than the free form: for example, the polarity coefficient for caffeic acid is (−1.76) and for its methylated compound is (+0.52) [23,24].
Quantitatively and qualitatively, polyphenols influence the composition and equilibrium of the beer matrix, and this influence depends not only on the structure and properties of the compounds themselves but also on external technological factors (temperature, pH, presence of microorganisms, and polar solvents) [25].
There is also an inverse relationship, where the plant matrix compounds affect the configuration of the polyphenols, which affects the sensory perception of beer and the role of polyphenols in this perception. It was found that the perceived bitterness of beer does not stand out in the overall experience of drinking beer, and the drink tastes softer because the bittering compounds (iso-α-resins and phenolic compounds) are associated with glucose residues in the beer matrix [26].
Phenolic compounds of plant objects play an important role. The representatives of phenolic compounds in cereals are phenolic acids, flavonoids, and lignans, both conjugates and aglycones [27]. The distribution of phenolic representatives in the grain structure is determined by their functional significance: phenolic acids (mainly ferulic, p-coumaric, and caffeic acids) are in the grain’s cortical layer, and provide antioxidant, antimutagenic effects [28,29]; flavonoids also have functional properties and are pigments of the grain [30]; lignans in connection with other compounds provide mechanical protection of grains from mechanical and other damages [31]. In terms of beer quality, they have an effect when mashing grain products under the condition of the high alkalinity of water. Most of the phenolic compounds in the husks are removed with the grain pellets at the wort filtration stage.
Endosperm polyphenols are more important in terms of their effect on beer quality. The endosperm of cereals contains aglycones form of ferulic, parabiosan, protocatechuic, gallic, and caffeic acids; the other representatives of phenolic compounds are associated with protein, carbohydrate, and other compounds in the whole grain and are released to some extent only during malting [32,33]. The main group of polyphenols in malted grains is the flavone-3-ol group, in particular (+)-catechin, (−)-epicatechin, prodelfinidin B3, and procyanidin B3, as previously reported [34].
In the structure of malt, phenolic compounds often appear as esters, glycosides, and complexes with polysaccharides (arabinoxylans, β-glucans, etc.) [35]. Hop lupulin glands contain a mixture of prenylated, geranylated, oxidized, and/or cyclized chalcones along with bitter acids and volatile oils [36], some of which are in the form of o-glycosides that determine their bioavailability [37].
By entering the wort through the process, malt and hop polyphenolic compounds interact with matrix structure-forming compounds (protein, carbohydrate, coloring, and aroma compounds, etc.). Therefore, it is important to consider the other biomolecules of the plant matrix of beer.
2. Hop Phenolic Compounds on Dry Hopping Beer Quality
Studies have shown that the effect of polyphenolic compounds on beer quality is related to many technological and raw material factors. There was a correlation between the change in nitrogen content, the level of iso-α-bitter hop resins, the content of β-glucan, and beer coloration, and it was 1.5–2 times higher in alcoholic beer compared to non-alcoholic beer.
The formation of the colloidal structure of dry hopping beer’s samples continued more intensively at the fermentation stage in the difference from the kettle hopping beer’s samples, due to the extraction of bitter, phenolic, and essential organic compounds from hop preparations, which is confirmed by other authors [44,49]. Researchers have noted the simultaneous extraction of α-bitter resins and the loss of iso-α-acid during dry-hopping through adsorption on yeast cells and hop preparation particles, the effect of the process temperature, and the pH change of beer in the upward direction [50,51]. The pH shift affects the intensity of beer’s color degree since melanoidins depend on the medium acidity [52], which is confirmed by our research. The binding of protein and bitter acids levels is explained by the direct extraction of hop resins during fermentation and their covalent binding to protein compounds through cysteine sites [53].
It is interesting to note that the form of binding of β-glucan molecules in beer can also occur with protein molecules, as was recently found, through the participation of Ca2+ ions, as shown in Figure 1 [54].
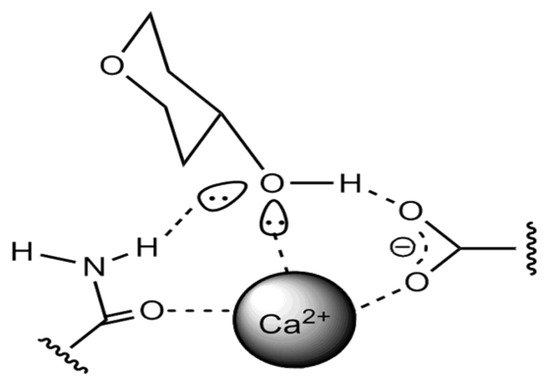
Figure 1. The protein–carbohydrate molecules connection in beer.
With regard to the interaction of glucans and other carbohydrates, there is a mechanism of binding to phenols through hydrogen bonds of hydroxyl groups and carboxyl groups of saccharide dextrins, such as glucans and arabinoxylans, etc., as well as through hydrophobic interactions [55]. This has significance in terms of representing the structure of the colloidal system concerning phenolic compounds, which have sensory, color, and structure-forming contributions in the evaluation of beer quality.
The study revealed a relationship between the iso-α-bitter resins content with isoxanthohumol and rutin to the greatest extent in the kettle hopping beer’s samples, and with isoxanthohumol and catechin in the dry hopping beer’s samples. In our opinion, the localization of the combined presence of bittering resins and phenolic compounds of hops is important [6], as well as the significance of the process of hop addition: hops adding during boiling tends to transform the phenolic complex of both hop and malt compounds more than the addition at the premalted stage; this may explain the importance of rutin in kettle hopping relative to the relationship with bittering resins and catechin in dry hopping conditions [48].
The formation of beer color is known to occur due to the presence of melanoidins, caramels, catechins, and riboflavin [56]. However, a correlation between rutin, catechin, and, among others, isoxanthohumol and beer’s color degree intensity under the kettle hoping technology was found in the study. The fact of preventing the isomerization of xanthohumol and isoxanthohumol in the presence of a prenylflavanoid and color can serve as confirmation of the connection between the isoxanthohumol and the color and the presence of melanoidins, caramels, and reductones of roasted malted and unsalted grain raw materials in the preparation of dark stouts [57,58].
The 2-hydroxyl group in the xanthohumol molecule is considered reactive, which provides its functional value [59].
Under the influence of temperature or oxygen, the 2-OH group is oxidized with the cleavage of the double bond, and xanthohumol is converted into isoxanthohumol, and melanoidins and reductones with their antiradical function prevent the binding of reactive oxygen species with the hydroxyl group of xanthohumol [60].
The catechins level in non-alcoholic beer was at 1.62 ÷ 3.96 mg/L, in alcoholic beer 2.96 ÷ 21.78 mg/L, and in dry hopped beer samples 6.44 ÷ 10.89 mg/L, values consistent with those previously reported [61,62]. We note that the obtained correlations confirm the close relationship between the beer’s color degree index and catechin content, which is associated with the chromophore properties of the molecule [63]. The correlation between catechins and proteins is based on the ability to form protein–phenol complexes as part of the antioxidant action through covalent bonds [64]. The relationship between isoxanthohumol and soluble nitrogen is based on the ability of prenylflavanoid to interact with NADN compounds during the active oxygen-binding reaction during intercellular interaction [61]. The close correlation between isoxanthohumol and catechins is based on the triple bond of isoxanthohumol–protein–catechin running in the colloidal system of beer. The intermolecular interactions of rutin and β-glucan were highlighted by us earlier and confirmed by other authors [55].
The color intensity in dry hopped beer depends on the same parameters as in kettle hopped beer samples, but the most significant were phenolic compounds: catechins, isoxanthohumol, and quercetin according to Equation (7). Since there is an active saturation of fermenting beer with hop compounds (phenolic, essential, and others), the influence of these compounds increases: common polyphenols had the most significant binding force in the evaluation of pair correlation with respect to all indicators.
It is interesting to note the effect of quercetin on the intensity of the beer’s color degree index. Under the presence of a polar extractant (ethyl alcohol digested by microorganisms), quercetin participates in the metabolism of yeast cells and is partially adsorbed on their surface due to mannan–glucan sites [48]. We note that the level of quercetin in the samples of dry hopped beer is slightly higher and is 13.04 ÷ 33.41 mg/L compared to the alcoholic kettle hopped beer—2.46 ÷ 31.02 mg/L (Table 2). Non-alcoholic beer contained the least amount of quercetin—0.75–11.02 mg/L, which indicates the loss of quercetin at the stage of beer release from alcohol.
Table 2. The phenol’s profile of beer samples.
| Sample Number | The Polyphenol Content in Samples, mg/L, Reliability Limit p ˂ 0.05 | ||||
|---|---|---|---|---|---|
| Total | Isoxanthohumol (IXG) | (+)Catechin (Ct) | Quercetin (Qv) | Rutin (Rt) | |
| 1NABK | (41.0 ± 3.7) * | 1.6 ± 0.02 | 1.24 ± 0.01 | 11.02 ± 0.10 | 3.53 ± 0.03 |
| 2NABK | 65.6 ± 6.0 | 1.1 ± 0.01 | 1.73 ± 0.01 | 2.78 ± 0.03 | 5.24 ± 0.05 |
| 3NABK | 82.0 ± 7.4 | 2.4 ± 0.02 | 3.96 ± 0.04 | 0.75 ± 0.01 | 6.56 ± 0.07 |
| 4ABK | 106.6 ± 9.6 | 2.2 ± 0.02 | 3.71 ± 0.04 | 9.90 ± 0.10 | 3.50 ± 0.04 |
| 5ABK | 98.4 ± 8.9 | 3.7 ± 0.04 | 2.97 ± 0.03 | 12.84 ± 0.10 | 7.39 ± 0.07 |
| 6ABK | 114.8 ± 10.3 | 4.5 ± 0.04 | 3.96 ± 0.04 | 12.09 ± 0.10 | 7.72 ± 0.08 |
| 7ABK | 139.4 ± 12.5 | 7.4 ± 0.07 | 21.78 ± 0.22 | 12.58 ± 0.10 | 6.05 ± 0.06 |
| 8ABK | 139.4 ± 12.5 | 3.8 ± 0.04 | 2.72 ± 0.03 | 11.94 ± 0.10 | 8.20 ± 0.08 |
| 9ABK | 172.2 ± 15.5 | 5.2 ± 0.05 | 21.78 ± 0.22 | 11.98 ± 0.10 | 7.83 ± 0.08 |
| 10ABK | 106.6 ± 9.6 | 3.0 ± 0.03 | 3.96 ± 0.04 | 12.83 ± 0.10 | 6.41 ± 0.06 |
| 11ABK | 123.0 ± 11.1 | 4.0 ± 0.04 | 4.21 ± 0.04 | 12.71 ± 0.10 | 8.74 ± 0.09 |
| 12ABK | 188.6 ± 11.3 | 2.2 ± 0.02 | 5.94 ± 0.06 | 2.46 ± 0.02 | 12.98 ± 0.13 |
| 13ABK | 287.0 ± 25.8 | 6.1 ± 0.06 | 8.42 ± 0.08 | 31.02 ± 0.30 | 1.96 ± 0.02 |
| 14ABK | 237.8 ± 21.4 | 3.5 ± 0.04 | 12.87 ± 0.13 | 22.10 ± 0.20 | 1.84 ± 0.02 |
| 15ABD | 147.6 ± 13.3 | 4.2 ± 0.04 | 7.43 ± 0.07 | 21.55 ± 0.20 | 2.80 ± 0.03 |
| 16ABD | 164.0 ± 14.8 | 3.4 ± 0.03 | 6.44 ± 0.05 | 13.04 ± 0.10 | 13.64 ± 0.14 |
| 17ABD | 213.2 ± 19.2 | 4.7 ± 0.05 | 8.91 ± 0.09 | 14.57 ± 0.10 | 4.43 ± 0.04 |
| 18ABD | 131.2 ± 11.8 | 4.6 ± 0.05 | 7.92 ± 0.08 | 24.20 ± 0.20 | 2.14 ± 0.02 |
| 19ABD | 192.7 ± 17.3 | 5.3 ± 0.05 | 10.89 ± 0.11 | 33.41 ± 0.30 | 2.38 ± 0.02 |
| 20ABD | 328.0 ± 29.5 | 9.4 ± 0.10 | 10.40 ± 0.10 | 20.20 ± 0.20 | 4.11 ± 0.04 |
It is known that quercetin also belongs to the class of antioxidants and prevents oxidation reactions in those biological systems where it is present [62]. Being involved in the processes of oxygen capture in the presence of yeast cells decreases its quantity [48], but this leads to an intensification of microbial metabolism, which leads to a greater accumulation of secondary fermentation products that indirectly affect all organic compounds of beer, including melanoidins, catechins, caramels, and others, leading to color index changes [46,47,65]. On the other hand, nitrogen molecules present in the system bind to molecules of phenolic compounds, which leads to their enlargement and subsidence [26,33,49,51]. These processes are confirmed by the correlation coefficients we have obtained.
Additionally, as a confirmation of sedimentation processes, we can say that the levels of β-glucan content confirm that sedimentation and removal of interacting carbohydrates, polyphenols, and nitrogenous compounds from the colloidal system occurs: the amount of β-glucan dextrins in alcohol beer was in the range 62.0 ÷ 240.5 mg/L, in dry hopped beer 31.0 ÷ 186.2 mg/L, and in waster nitrogen 306.8 ÷ 1185.0 and 560.3 ÷ 1075.0 mg/L, respectively; the level of polyphenols in dry hopped beer was higher—131.2 ÷ 328.0 mg/L, and in kettle hopped beer samples was lower—98.4 ÷ 237.8 mg/L (Table 3). Of course, different raw materials and beer’s origin extract content must be taken into consideration.
Table 3. The beer’s samples characteristics.
| Sample Number | The Content in Samples, mg/L, Reliability Limit p ˂ 0.05 | |||||
|---|---|---|---|---|---|---|
| Alcohol, vol% | Original Extract, °P | β-Glucan (Gl) | Iso-α-Humulon (IBU) (IH) | Soluble Nitrogen (SN) | Color, °EBC | |
| 1NABK | (0.49 ± 0.05) * | 7.0 ± 0.70 | 65.0 ± 4.6 | 11.8 ± 0.06 | 300.4 ± 12 | 6.75 ± 0.20 |
| 2NABK | 0.48 ± 0.05 | 7.5 ± 0.70 | 69.8 ± 4.9 | 21.6 ± 0.11 | 439.8 ± 18 | 5.00 ± 0.15 |
| 3NABK | 0.48 ± 0.05 | 7.8 ± 0.80 | 108.6 ± 7.6 | 13.5 ± 0.07 | 630.6 ± 25 | 7.50 ± 0.22 |
| 4ABK | 4.6 ± 0.40 | 10.7 ± 1.00 | 62.1 ± 4.3 | 9.7 ± 0.05 | 459.4 ± 20 | 5.25 ± 0.16 |
| 5ABK | 4.5 ± 0.40 | 10.8 ± 1.00 | 124.1 ± 8.7 | 6.3 ± 0.03 | 445.4 ± 25 | 5.75 ± 0.17 |
| 6ABK | 5.1 ± 0.50 | 11.0 ± 1.00 | 62.0 ± 4.3 | 12.9 ± 0.06 | 984.0 ± 40 | 7.50 ± 0.22 |
| 7ABK | 4.7 ± 0.40 | 11.3 ± 1.00 | 77.6 ± 5.4 | 24.4 ± 0.12 | 1185.0 ± 47 | 106.3 ± 3.19 |
| 8ABK | 4.8 ± 0.50 | 11.6 ± 1.00 | 62.1 ± 4.3 | 26.3 ± 0.13 | 823.6 ± 33 | 6.75 ± 0.20 |
| 9ABK | 4.5 ± 0.40 | 11.8 ± 1.00 | 75.6 ± 5.3 | 12.3 ± 0.06 | 980.0 ± 40 | 25.0 ± 0.75 |
| 10ABK | 5.0 ± 0.50 | 11.9 ± 1.00 | 128.0 ± 9.0 | 14.1 ± 0.07 | 306.8 ± 12 | 5.25 ± 0.16 |
| 11ABK | 5.2 ± 0.50 | 12.0 ± 1.00 | 120.3 ± 8.4 | 12.2 ± 0.06 | 743.0 ± 30 | 7.25 ± 0.21 |
| 12ABK | 5.3 ± 0.50 | 12.8 ± 1.00 | 240.5 ± 16.8 | 4.9 ± 0.02 | 972.1 ± 39 | 9.50 ± 0.29 |
| 13ABK | 8.1 ± 0.80 | 16.5 ± 1.50 | 93.1 ± 6.5 | 26.5 ± 0.13 | 888.0 ± 36 | 5.25 ± 0.16 |
| 14ABK | 9.2 ± 0.90 | 18.6 ± 1.50 | 96.2 ± 6.7 | 12.7 ± 0.06 | 854.4 ± 34 | 17.5 ± 0.53 |
| 15ABD | 4.6 ± 0.40 | 10.0 ± 1.00 | 31.0 ± 2.2 | 29.1 ± 0.15 | 560.3 ± 22 | 9.50 ± 0.29 |
| 16ABD | 4.9 ± 0.50 | 12.0 ± 1.00 | 93.1 ± 6.5 | 28.7 ± 0.14 | 935.7 ± 37 | 5.25 ± 0.16 |
| 17ABD | 6.6 ± 0.70 | 14.5 ± 1.00 | 186.2 ± 13.0 | 32.4 ± 0.16 | 823.6 ± 33 | 12.5 ± 0.38 |
| 18ABD | 5.9 ± 0.60 | 15.0 ± 1.50 | 155.2 ± 10.9 | 58.3 ± 0.30 | 767.6 ± 30 | 16.5 ± 0.50 |
| 19ABD | 5.9 ± 0.60 | 16.0 ± 1.50 | 74.5 ± 5.2 | 42.6 ± 0.21 | 798.4 ± 32 | 5.25 ± 0.16 |
| 20ABD | 7.7 ± 0.80 | 17.5 ± 1.50 | 108.6 ± 7.6 | 76.2 ± 0.36 | 1075.7 ± 43 | 17.0 ± 0.51 |
The evaluation of the colloidal matrix compounds effect on the organoleptic profile of beer samples showed differences (Figure 2, Table 4).
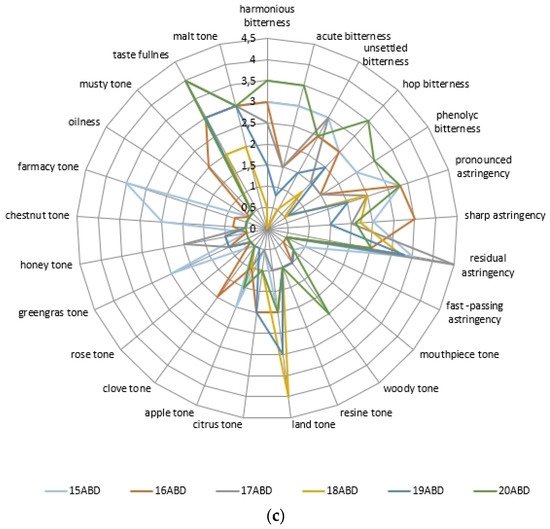
Figure 2. The beer’s sample descriptor analysis data: with kettle hopping non-alcoholic samples (a), alcoholic samples (b), and dry hopping samples (c).
Table 4. The beer samples correlation–regression indicators.
| Indicators | The Beer’s Samples Group | |
|---|---|---|
| Kettle Hoped | Dry Hoped | |
| Bitterness Descriptor: harmonious bitterness (hb) |
||
| significant compounds according to the elasticity coefficient (EC) | IH (EC = 0.86); IXG (EC = −0.84); Rt (EC = 0.57) |
SN (EC = 1.73); Qv (EC = −1.34); IH (EC = −1.04); Gl (EC = −0.79); IXG (EC = 0.76) |
| the descriptor influencing factors and their correlation coefficient (Rc) | IXG/SN (Rc = 0.73); IH/Ct (Rc = 0.72); Ct/SN (Rc = 0.69); hb/Rt (Rc = 0.62) IXG/Qv (Rc = 0.58); hb/Qv (Rc = −0.52) hb-Rt/SN (Rc = 0.71); hb-Rt/IXG (Rc = 0.66) hb-Rt/Ct (Rc = 0.64); hb-Rt/Gl (Rc = 0.56) hb-Qv/SN (Rc = 0.50) |
IH/IXG (Rc = 0.84); IXG/Ct (Rc = 0.75) IH/SN (Rc = 0.64); IH-IXG/hb (Rc = 0.96) hb-IXG/IH (Rc = 0.89); hb-IH/IXG (Rc = −0.85) IXG-Ct/hb (Rc = 0.77); IH-SN/hb (Rc = 0.71) hb-IXG/Rt (Rc = 0.69); IXG-SN/hb (Rc = 0.67) hb-IXG/Qv (Rc = 0.63); Qv-Gl/hb (Rc = −0.63) Ct-Rt/hb (Rc = −0.63); hb-IXG/Gl (Rc = 0.55) |
| the general correlation coefficient (GCC) | 0.78 | 1.0 |
| the general determination coefficient (GDC) | 0.62 | 1.0 |
| the unreported compounds contribution, % | 38.4 | 0.0 |
| Descriptor: acute bitterness (ab) | ||
| significant compounds according to the elasticity coefficient (EC) | Gl (EC = −0.57); IXG (EC = −0.54) Rt (EC = 0.52); SN (EC = 0.51) |
SN (EC = −4.23); Ct (EC = 2.12) Qv (EC = −1.94); IXG (EC = 1.72) IH (EC = 0.77); Gl (EC = −0.64) |
| the descriptor influencing factors and their correlation coefficient (Rc) | Ct/SN (Rc = 0.69); IXG/SN (Rc = 0.62) ab/IH (Rc = 0.61); ab-IH/IXG (Rc = 0.54) ab-IH/Qv (Rc = 0.59); ab-IH/Ct (Rc = 0.52) IXG-Ct/Gl (Rc = 0.73); IXG-Ct/IH (Rc = 0.73) IXG-Ct/Rt (Rc = 0.72); IXG-Ct/Qv (Rc = 0.72) |
ab/IXG (Rc = 0.72); IH/IXG (Rc = 0.84) IXG/Ct (Rc = 0.74); IXG/SN (Rc = 0.74) IH/SN (Rc = 0.64); IH-IXG/ab (Rc = 0.91) IXG-Ct/ab (Rc = 0.79); IXG-SN/ab (Rc = 0.71) IH-SN/ab (Rc = 0.69) |
| the general correlation coefficient (GCC) | 0.89 | 1.0 |
| the general determination coefficient (GDC) | 0.80 | 1.0 |
| the unreported compounds contribution, % | 19.6 | 0.0 |
| Descriptor: hop bitterness (hb) | ||
| significant compounds according to the elasticity coefficient (EC) | IXG (EC = 0.59); GL (EC = −0,29) Qv (EC = −0,22); Rt (EC = 0.20) SN (EC = −0.20) |
SN (EC = −3.59); IH (EC = −1.23) Gl (EC = −1.13); Ct (EC = −1.02) IXG (EC = −0.92) |
| the descriptor influencing factors and their correlation coefficient (Rc) | hb/Qv (Rc = 0.74); hb/IXG (Rc = 0.58) IXG/SN (Rc = 0.79); IXG/Ct (Rc = 0.72) IH/Gl (Rc = 0.50); hb-IXG/Rt (Rc = 0.76) hb-Qv/IH (Rc = −0.71); hb-IXG/Gl (Rc = −0.57); hb-IXG/IH (Rc = 0.52) |
IH/IXG (Rc = 0.80); IXG/Ct (Rc = 0.77) IXG/SN (Rc = 0.76); IH/SN (Rc = 0.62) hb-IH/IXG (Rc = −0.87); hb-IXG/IH (Rc = 0.81) IXG-Ct/hb (Rc = 0.79); hb-Qv/Gl (Rc = −0.77) hb-IXG/Rt (Rc = 0.61); Qv-Gl/hb (Rc = 0.73) IH-SN/hb (Rc = 0.72); IXG-SN/hb (Rc = 0.71) |
| the general correlation coefficient (GCC) | 0.84 | 1.0 |
| the general determination coefficient (GDC) | 0.70 | 1.0 |
| the unreported compounds contribution, % | 29.9 | 0.0 |
| Descriptor: phenolic bitterness (pb) | ||
| significant compounds according to the elasticity coefficient (EC) | IXG (EC = −0.55): Qv (EC = 0.52) Rt (EC = 0.55) |
SN (EC = 2.52); Qv (EC = −2.31); Gl (EC = −1.24); Rt (EC = −0.80) |
| the descriptor influencing factors and their correlation coefficient (Rc) | pb/Qv (Rc = 0.43); IXG/SN (Rc = 0.73) IXG/Ct (Rc = 0.72); IXG/Rt (Rc = 0.59) Ct-SN/pb (Rc = 0.64); Rt-Gl/pb (Rc = 0.63) IH-Gl/pb (Rc = −0.59); pb-Qv/Rt (Rc = 0.56); pb-Qv/Gl (Rc = 0.51) |
IH/IXG (Rc = 0.78); IH/Ct (Rc = 0.52) IH-IXG/pb (Rc = 0.90); IXG-Ct/pb (Rc = 0.85); pb-IH/IXG (Rc = −0.71); IXG-SN/pb (Rc = 0.70) Qv-Rt/pb (Rc = 0.69); pb-IXG/Ct (Rc = 0.64) IH-SN/pb (Rc = 0.59) |
| the general correlation coefficient (GCC) | 0.81 | 1.0 |
| the general determination coefficient (GDC) | 0.65 | 1.0 |
| the unreported compounds contribution, % | 34.5 | 0.0 |
| Astringency Descriptor: pronounced astringency (pa) |
||
| significant compounds according to the elasticity coefficient (EC) | Gl (EC = −0.58); Rt (EC = 0.55); Ct (EC = 0.50); IXG (EC= −0.46) |
SN (EC = −6.93); Ct (EC = 6.00); IH (EC = 3.14) Qv (EC = −2.36); Gl (EC= −1.26); Rt (EC = 1.17) |
| the descriptor influencing factors and their correlation coefficient (Rc) | pa/Rt (Rc = 0.55); pa-IXG/Rt (Rc = 0.56) pa-Qv/IXG (Rc = −0.64); pa-Rt/Gl (Rc = 0.64); Ct-SN/pa (Rc = 0.67) |
pa-IXG/Ct (Rc = 0.89); pa-Qv/IH (Rc = 0.88); pa/Ct (Rc = −0.54); pa/Qv (Rc = −0.74); pa-Ct/SN (Rc = −0.70); IXG-SN/pa (Rc = 0.73) pa-IH/Ct (Rc = 0.69); pa/Rt (Rc = 0.50) |
| the general correlation coefficient (GCC) | 0.85 | 1.0 |
| the general determination coefficient (GDC) | 0.72 | 1.0 |
| the unreported compounds contribution, % | 27.9 | 0.0 |
| Descriptor: sharp astringency (sa) | ||
| significant compounds according to the elasticity coefficient (EC) | SN (EC = 1.08); IXG (EC = −0.97) Rt (EC = 0.85); Ct (EC = 0.72) |
Ct (EC = 7.26); SN (EC = −6.36); IH (EC = 2.34); IXG (EC= −1.61); Qv (EC = −1.78); Rt (EC = 1.30) |
| the descriptor influencing factors and their correlation coefficient (Rc) | sa-Ct/SN (Rc = 0.68); sa-IXG/Ct (Rc = 0.60) Ct-SN/sa (Rc = 0.83); IH-Ct/sa (Rc = 0.82) Rt-Gl/sa (Rc = 0.62); IH-Gl/sa (Rc = 0.56) |
sa/Gl (Rc = 0.77); sa-Gl/IH (Rc = 0.91); sa-Gl/Ct,Qv, Rt,SN (Rc = 0.86); sa-IH/Gl (Rc = −0.80); sa-Gl/IXG (Rc = 0.79); IXG-Ct/sa (Rc = 0.76); IXG-SN/sa (Rc = 0.75) |
| the general correlation coefficient (GCC) | 0.85 | 1.0 |
| the general determination coefficient (GDC) | 0.72 | 1.0 |
| the unreported compounds contribution, % | 28.4 | 0.0 |
| Descriptor: residual astringency (ra) | ||
| significant compounds according to the elasticity coefficient (EC) | IXG (EC= 0.69); IH (EC = −0.66) Gl (EC = −0.62); SN (EC = 0.53) Qv (EC = −0.52) |
SN (EC = 65.56); Ct (EC = −77.84); IH (EC = −33.37); IXG (EC= 23.67); Qv (EC = 26.106); Rt (EC = −11.44) |
| the descriptor influencing factors and their correlation coefficient (Rc) | IXG-SN/ra (Rc = 0.73); IXG-Ct/ra (Rc = 0.72) IH-IXG/ra (Rc = 0.71); IXG-Qv/ra (Rc = 0.65) |
IH-IXG/ra (Rc = 0.83); ra-SN/Gl (Rc = −0.79); Qv-Rt/ra (Rc = −0.77); SN-Gl/ra (Rc = 0.75); ra-IH/Rt (Rc = −0.70); Ct-Qv/ra (Rc = 0.69); IXG-Ct/ra (Rc = 0.68); ra-Rt/Qv (Rc = −0.66); ra-Gl/IH (Rc = 0.65); IH-Rt/ra (Rc = −0.64); Ct-Rt/ra (Rc = −0.64); ra-IH/Ct (Rc = −0.55) |
| the general correlation coefficient (GCC) | 0.61 | 1.0 |
| the general determination coefficient (GDC) | 0.37 | 1.0 |
| the unreported compounds contribution, % | 62.8 | 0.0 |
By evaluating the score of the kettle hopped beer descriptors, it was shown that isohumulone, isoxanthohumol, and rutin respond to harmonious bitterness, with isoxanthohumol and rutin correlated with soluble nitrogen content, which equalizes the sensation of bitterness (Table 4). We note that the descriptor describing harmonic bitterness contributes more or less to all phenolic compounds.
The contribution of isomerized forms of α-acids has been widely studied. Thus, iso-α-acids are mainly considered responsible for beer bitterness [66], and isomerization affects the compression of the acyloid ring, which allows the perceived bitterness from isomerized resins [67].
The question of understanding the main compounds responsible for bitterness is still open [67], but the influence of different forms of hop iso-α-acids is still held [68,69]. The phenomenon of cyclization of isomerized acids without oxygen with the participation of protons with the formation of tri- and tetracyclic decomposition products, as well as aldehydes, which contributed to the formation of persistent sharp bitterness [67]. There is an opinion that undesirable tones of bitterness come from the products of autodegradation of isomerized hydroperoxy- and hydroxyl-allo-iso-alpha-acid resins [70]. It has been noted that the same BU units can characterize different levels of sensory perception of bitterness at the raw material level [71], which suggests a complex organization of sensory perception of beer bitterness and confirms our findings.
The harmonic bitterness of dry hopped beer samples (Table 4) differs from the kettle hopped beer one mainly in the phenolic profile (isoxanthohumol, rutin, and quercetin) with the influence of soluble nitrogen and dextrins β-glucan. The importance of organic biomolecules in the perception of the sensory descriptor of harmonic bitterness has not been assessed before, based on the studied literature, but researchers confirm the importance of the grain genetics role in the beer’s taste, which is one of the main sources of protein and carbohydrate compounds [72]. On the other hand, there is evidence that the addition of a pectin solution as a source of carbohydrate smoothed the taste of the non-harmonic profile of the beer [73]. In the perception of harmonic bitterness, therefore, the attributes of the beer’s test fullness (soluble nitrogen and starchy and nonstarchy nature dextrins) have a great influence [74].
In the evaluation of the acute bitterness descriptor, which is usually correlated with the isomerized and biotransformed hop resins complex presence [67,68,69,70], the largest contribution in terms of the correlation of the evaluation with specific indicators were dextrin molecules β-glucan, isoxanthohumol, and rutin (Table 4), which correlates with acute bitterness in dry hopped beer’s samples but with a wider range of phenolic compounds. Carbohydrate molecules serve as buffer systems for the expression of certain descriptors expressing the bitterness of beer in the kettle hopped beer samples. In dry hopping beer, a significant contribution was made to prenylflavanoid and other forms of phenolic compounds, as well as soluble nitrogen (Table 4). This is consistent with the previously obtained data, when it was shown that the conditions of dry hopping contribute to the transfer to the fermented beer maximum amounts of procyanidin B3, catechin, and caffeic acid [75], which may lead to the formation of protein–polyphenolic associates, which it will cause turbidity, as well as isomerization reactions of xanthohumol into isoxanthohumol [14].
In the evaluation of hop bitterness, there is an influence of carbohydrate dextrins in the case of kettle hopped beer’s samples and soluble nitrogen in the case of dry hopping beer, along with the influence of bittering and phenolic compounds, quercetin and rutin, in classical hopping and catechin in cold hopping (Table 4).
Phenolic bitterness is important from the point of view of phenolic profile evaluation, since, as it is known, phenolic compounds are very labile and changes in parameters (O2, pH, concentrations of potential associate compounds, temperature, etc.) can cause quantitative and qualitative modifications that affect beer taste profile [12,14,26,75].
For this reason, compounds with antioxidant activity are present in the significant factors of the phenolic bitterness descriptor (Table 4), and only partial correlation factors indicate a broader profile of compounds that form the phenolic oxidation status of beer.
The astringency descriptor is associated in brewers with the content of grain phenols (tannins, catechins) that pass into the wort during mashing [6,12]. The pH of the environment is important for the equilibrium state of these compounds since it is known that the structure at pH closer to alkaline zones leads to the transformation and further degradation of catechins and catechin oligomers, which affects the color, taste, and appearance of beer [21]. It is noted that catechin derivatives were found in beer in the form of (+)catechin and (−)epigallocatechin in low amounts [76,77]. Some data suggest the significance of flavonoid configurations with respect to the sensory properties they present. For example, epicatechin is more bitter and astringent than its chiral isomer catechin [78,79]. The position of the double bond, the identity of the monomeric units, and the introduction of extrinsic radicals equally affect the astringency and bitterness of dimeric or trimeric molecules [80,81], which makes it more understandable that dextrins and nitrogenous molecules participate in the formation of different shades of bitterness and astringency.
On the other hand, mutual suppression of bitterness and sweetness in mixtures has been noted [82,83]. The increase in sweetness or viscosity related to dextrins of carbohydrates of both starchy and nonstarchy nature reduced the intensity of bitterness in vermouth [84], astringency in red wine [85,86,87,88,89].
The astringency descriptor was further divided into acute and residual since the perceptibility of taste shades depends not only on quality but also on human physiology [90].
The sharp astringency was evaluated in terms of the greater contribution of isoxanthohumol, rutin, and catechin and balanced with nitrogenous compounds in the kettle hopped beer, whereas in dry hopped beer, isohumulone and quercetin were added to the same compounds (Table 4), which is justified by the largest amount a variety of phenolic compounds that pass into the beer during fermentation [75].
It is necessary to note that the correlation coefficients indicate the influence on each other with respect to the contribution to the sharp tartness of catechin and soluble nitrogen, as well as isohumulone and catechin, which speaks in favor of the inherent ionic or covalent bond between catechin, soluble nitrogen, and iso-α-acid in the colloidal structure of the classical beer. In dry hopped beer, the greatest influence on the descriptor was exerted by β-glucan with catechin, rutin, quercetin, and soluble nitrogen, and the influence of catechin-isoxanthomol-soluble nitrogen agglomerate was inherent (Table 4).
The residual astringency (Table 4) differed from the sharp one in classic beer by the absence of rutin and catechin, and in dry hopped beer, there were no differences in the connections responsible for this descriptor.
Evaluating the regression coefficients, it can be noted that the combination of bitter resins, prenylflavanoid, and catechin are more responsible for the sharp astringency, and the residual bitterness is caused by catechin, quercetin, and rutin, associated with soluble nitrogen and β-glucan dextrins.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27030740
This entry is offline, you can click here to edit this entry!
