Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Gremlin-1 is a secreted cystine-knot protein that acts as an antagonist of bone morphogenetic proteins (BMPs), and as a ligand of heparin and the vascular endothelial growth factor receptor 2 (VEGFR2), thus regulating several physiological and pathological processes, including embryonic development, tissue fibrosis and cancer. Gremlin-1 exists both as a monomeric and dimeric protein. Unlike the dimeric protein, the monomeric form of gremlin-1 antagonizes VEGFR2
- gremlin-1
- dimer
- cystine-knot protein
- HEK293T expression system
1. Introduction
The gremlin-1 gene is highly conserved across evolution and, in humans, it encodes for a 184 amino acid long protein, whose theoretical molecular weight is 20.682 Da [1]. Gremlin-1 (hereinafter referred to as gremlin) is a highly basic polypeptide (pI = 9.53), containing 7.61% arginine, 8.7% lysine and 2.17% histidine residues. A signal peptide drives gremlin secretion. Gremlin is found in the endoplasmic reticulum and associated with the cell surface, where it binds to heparan sulfate proteoglycans (HSPGs) once it has been secreted [2]. Gremlin contains three serine residues, which may undergo phosphorylation (Ser77, Ser140 and Ser142). Ser77 was confirmed to be phosphorylated [3]. Putative tyrosine phosphorylation sites are also present. Gremlin is N-glycosylated on Asn42 [2]. However, the biological role of such modifications is not known.
The C-terminal region of Gremlin contains nine Cys residues (CX13CX8-9CX3CX14-18CX2CX13CX15-18CXC) that show the characteristic spacing of the cystine-knot protein consensus sequence [4]. Crystallographic analyses confirmed that gremlin has the typical folding of cystine-knot proteins, with six Cys residues involved in the formation of the cystine-knot and two Cys residues engaged in an intra-chain disulfide at the fingertips, thus resembling the structure of TGF-β family members [5]. Even though gremlin forms a compact, head-to-tail, non-covalent homodimer in the crystal structure, experimental evidence shows that gremlin forms disulfide-bound homodimers through Cys141 [6].
Gremlin is a pleiotropic protein that belongs to the Cerberus/Dan-related gene family of bone morphogenic protein (BMP) antagonists. By binding and sequestering BMP2/4/7 into inactive complexes, it orchestrates BMP signaling during embryonic development, tissue fibrosis and cancer [7,8,9]. Gremlin is also endowed with BMP-independent activities [10,11,12,13]. Among these, it binds the vascular endothelial growth factor receptor 2 (VEGFR2), eliciting pro-inflammatory and pro-angiogenic responses in tumors and kidney disease [11,12,13,14]. The activation of VEGFR2 on the surface of endothelial cells (ECs) by gremlin triggers EC proliferation, migration, and the formation of endothelial sprouts in vitro and in vivo [11]. We have previously shown that the VEGFR2-dependent activity of gremlin is modulated by its oligomeric state. While both states (monomeric and dimeric) bind to VEGFR2, only the dimeric form activates the receptor and angiogenesis. Monomeric gremlin, on the other hand, has been found to act as a VEGFR2 antagonist [6,15]. In addition, the pro-angiogenic activity of gremlin is mediated by its interaction with heparin and HSPGs on cell surfaces, occurring through twelve non-contiguous arginine and lysine residues [16,17]. The high affinity for heparin has previously been exploited for the isolation of gremlin from the culture medium of ECs [3]. Although the BMP antagonist activity of gremlin remains its best characterized feature to date, we now have clear evidence that the biological role of this protein results from a combination of all its distinct activities.
Recombinant gremlin from different sources (e.g., expressed in E. coli and refolded, CHO or mouse myeloma cells) has been characterized, providing valuable insight into the structure and mechanism of action of this protein. However, currently available methods for the expression and purification of recombinant gremlin are not best suited to consistently obtain dimeric gremlin that retains full biological activity. Consistent with this observation, a recent study failed to demonstrate that gremlin produced in mouse myeloma cells activates VEGFR2, while being a strong BMP-inhibitor [18]. This is not surprising, as gremlin dimerization is necessary for VEGFR2 activation but not for BMP inhibition [6] and considering that most of the available recombinant gremlin exists in both monomeric and dimeric states, with relatively low dimer vs. monomer ratios which can vary from batch to batch.
2.Production and Biochemical Characterization of Dimeric Recombinant Gremlin-1
2.1. Expression of Recombinant Gremlin in HEK293T Cells
Due to the complex structure of gremlin (i.e., intra and inter-molecular disulfides; N-glycosylation), the mammalian HEK293T expression system was chosen to produce the recombinant protein. Adherent HEK293T cells were transiently transfected with a pcDNA3 vector harboring the cDNA of rat gremlin (including the signal peptide-encoding sequence) added with a C-term hexa-histidine tag. As assessed by Western blot (WB), transfected HEK293T cells secreted significant amounts of gremlin (~0.8 μg/mL) into their supernatant. To increase gremlin expression, 6 mM sodium butyrate, a known inhibitor of histone deacetylases and cell proliferation [19,20], was added to the HEK293T cells. Under these circumstances, the yield increased to ~3μg/mL. Gremlin released by HEK293T cells ran in an SDS-PAGE under reducing conditions as two bands of ~24–27 kDa, corresponding to the glycosylated (~30–45%) and non-glycosylated (~70–55%) forms of the protein (Figure 1A,B). Accordingly, a single ~24 kDa band was observed following treatment of recombinant gremlin with the glycosidase PNGaseF (Figure 1C). Following transfection, the peak of protein expression was reached at 24 h post-transfection (Figure 1D).
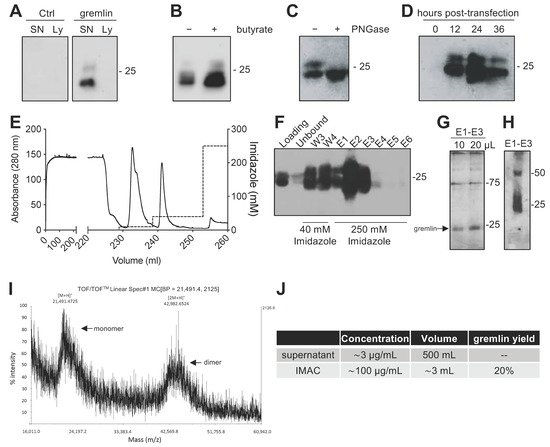
Figure 1. Expression and IMAC purification of recombinant gremlin in HEK293T cells. (A) WB analysis of gremlin expression in the supernatant (SN) or the lysate (Ly) of HEK293T cells transiently transfected with pcDNA3-Gremlin-1-HisTag (gremlin) or empty vector (ctrl). (B) WB analysis of gremlin expression in the supernatant of HEK293T cells in the absence or the presence of 6 mM sodium butyrate. (C) WB analysis of gremlin expressed and secreted by HEK293T cells in the absence or the presence of PNGaseF glycosidase. (D) WB analysis of gremlin expressed and secreted by HEK293T cells 0–36 h post-transfection. (E–H) IMAC of recombinant gremlin expressed in the supernatant of HEK293T cells. The chromatogram is shown in (E). Fractions were analyzed by WB for gremlin presence (F), or by silver staining (G). Non-reducing SDS-PAGE followed by anti-gremlin WB of purified gremlin is shown in (H). (I) MALDI-TOF/TOF–MS spectrum of IMAC-purified recombinant gremlin. Arrows indicate the two species of gremlin that were detected. (J) Total yield of IMAC gremlin purification. Data for one representative experiment of at least 3 independent repeats are shown.
2.2. Expression of Recombinant Gremlin in HEK293T Cells Followed by Purification via IMAC Yields Gremlin Both in the Monomeric and Dimeric States
Next, we attempted a medium-scale gremlin production followed by a first-step purification by ion metal affinity chromatography (IMAC). HEK293T cells were transiently transfected in a medium containing 0.5% FCS, and the supernatant (~500 mL) was collected 24 h post transfection, dialyzed against phosphate buffer and loaded onto a Ni-NTA column. As shown in Figure 1E,F, recombinant gremlin eluted from the nickel column at 250 mM imidazole. Although some gremlin was lost in the 40 mM imidazole washing step, purified gremlin was present in the eluted fraction (~24 kDa band) in the absence of significant amounts of contaminant proteins, as assessed by gel silver staining (Figure 1G). Non-reducing SDS-page followed by anti-gremlin WB showed that gremlin eluted both in a monomeric and ~50 kDa disulfide-bound homodimeric forms (Figure 1H). This was further confirmed by MALDI-TOF/TOF–MS (Figure 1I). The main peak, with an m/z value of approximately 21,500, represented the single charge of the monomer of gremlin, while the m/z value of about 43,000 represented the single charge of gremlin dimer. The IMAC purification step had an overall yield of 20% (Figure 1J). Similarly, IMAC-purified, 10xhis-tagged gremlin, expressed in murine myeloma cells, existed in both monomeric and dimeric states. The relative amount of dimeric protein differed significantly from batch to batch, and in some cases only reached 1.5% of total gremlin (Figure S1).
2.3. IMAC-Purified Gremlin Exhibits Full BMP Antagonist Activity and Only Mild VEGFR2 Activation Capacity
The IMAC-purified gremlin was tested for its biological activities. In a first set of experiments, to verify its BMP antagonist activity, hepatocarcinoma HepG2 cells were stimulated with BMP4 in the absence or the presence of increasing doses of IMAC-purified gremlin and assessed for phospho-SMAD1/5/8 levels by WB. As expected, IMAC-purified gremlin induced a dose-dependent inhibition of BMP4-driven SMAD phosphorylation (Figure 2A). We obtained similar results with all different IMAC gremlin preparations (data not shown). On the contrary, not all batches of IMAC gremlin induced a significant activation of VEGFR2 or of the VEGFR2-dependent biological activities in the human umbilical vein endothelial cells (HUVEC) (data not shown). Figure 2B,C shows the weak activation of VEGFR2 obtained with one of the different batches of purified IMAC gremlin that were tested. Indeed, VEGFR2 and angiogenesis were only activated when high doses of IMAC-purified gremlin (100–200 ng/mL) were used.
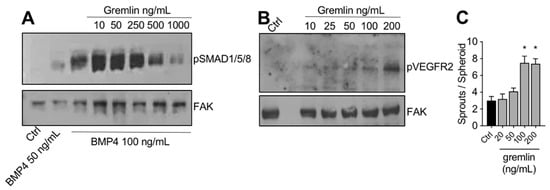
Figure 2. IMAC-purified gremlin retained full BMP-dependent activity and only mild VEGFR2 activation capacity. (A) WB analysis of phospho-SMAD1/5/8 levels in HepG2 cells stimulated with BMP4 in the presence or the absence of indicated doses of IMAC-purified gremlin. FAK, loading control. (B) WB analysis of phospho-VEGFR2 levels in HUVE cells stimulated with increasing doses of IMAC-purified gremlin. FAK, loading control. (C) HUVE cells sprouting assay in the absence or the presence of increasing doses of IMAC-purified gremlin. Data are expressed as mean ± SEM. *, p < 0.05 Student’s t test vs. untreated cells. Data from one representative experiment of at least 3 independent repeats are shown.
2.4. Purification of Fully Active, Dimeric Gremlin by Heparin-Affinity Chromatography
The varying and low ability of the IMAC-purified gremlin to activate VEGFR2 suggests the possibility that some of the protein may be present in an unfolded, biologically inactive form. This prompted us to introduce an additional purification step. We exploited heparin-affinity chromatography, as the heparin binding domain of gremlin consists of a non-linear 12-aa sequence [17] that allowed us to isolate the correctly folded and fully active gremlin protein. IMAC-purified gremlin was loaded on a heparin-sepharose (HepSep) column and eluted with a 0.2 to 1.2 M NaCl step gradient. WB analysis demonstrated that the 0.7 M NaCl fraction contained gremlin monomers together with dimers probably not correctly folded (Figure 3A), whereas gremlin eluting at higher ionic strength (0.8–0.9 M NaCl) ran on a non-reducing SDS-PAGE as a ~50 kDa dimer. The net yield of this second purification step was ~5% (Figure 3B).
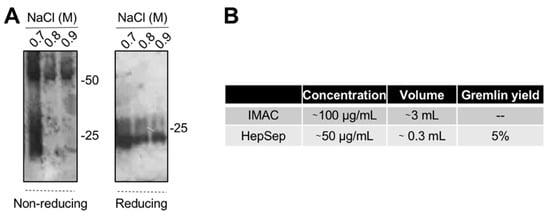
Figure 3. Heparin-affinity chromatography allows isolating pure dimeric recombinant gremlin. IMAC-purified gremlin was subjected to a second step of purification through heparin-affinity chromatography. (A) WB analysis under non-reducing and reducing conditions of fractions eluted from the heparin-affinity chromatography. (B) Total yield of gremlin purification through heparin-affinity chromatography. Data from one representative experiment of at least 3 independent repeats are shown.
We next measured the bioactivity of HepSep-purified dimeric gremlin. As shown in Figure 4A, HepSep gremlin blocks the BMP4-dependent activation of the hepcidin promoter in a luciferase reporter assay, at an extent similar to that of IMAC-purified gremlin. On the contrary, dimeric gremlin induced a significantly more potent response than IMAC-purified gremlin in terms of VEGFR2 activation, measured as phosphorylated receptor accumulated on the ventral plasmatic membrane (VPM), and in terms of proliferation of HUVECs (Figure 4B,C). These results are consistent with our previous findings showing that monomeric and dimeric gremlin antagonize BMPs in a similar manner, while only dimeric gremlin induces the full activation of ECs via VEGFR2 [6].
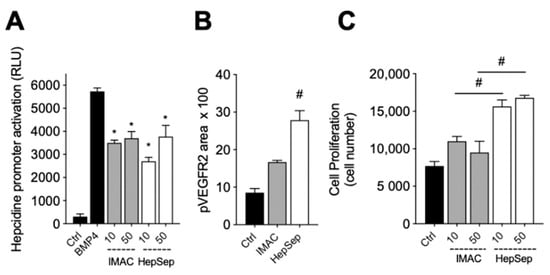
Figure 4. Pure dimeric gremlin exhibits strong biological activity. (A) Activation of Hepcidin promoter in a luciferase reporter assay upon BMP4 stimulation (50 ng/mL). When indicated, BMPs were pre-incubated in the absence, or presence, of the indicated concentration (ng/mL) of IMAC-purified or dimeric heparin-sepharose (HepSep)-purified gremlin. (B) pVEGFR2 accumulation into the VPM of GM7373-VEGFR2 endothelial cells adherent to IMAC-purified or dimeric HepSep-purified gremlin (dishes were coated with 2 µg/mL of recombinant proteins). (C) Cell proliferation of HUVE cells stimulated with increasing doses (ng/mL) of IMAC- or HepSep-purified gremlin. Data are expressed as mean ± SEM of three independent experiments. *, p < 0.05 Student’s t test vs. BMP-treated cells. #, p < 0.05 Student’s t test vs. gremlin IMAC-treated cells.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031151
This entry is offline, you can click here to edit this entry!
