Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Arsenic is a metalloid with natural and anthropogenic sources and its inorganic form is toxic to humans. Rice is highly consumed worldwide and is prone to arsenic contamination.
- arsenic
- rice
- environmental contaminants
- estimated daily intake
- risk assessment
1. Introduction
Modern agriculture practices have increased productivity, but at high environmental costs. The increased use of chemical compounds has led to serious pollution problems across the planet, causing different environmental issues, such as the contamination of metals and metalloids in food chains, jeopardizing food safety. Food and water are the most common sources of human exposure to metals and metalloids [1][2].
Among these toxic compounds is arsenic, a metalloid or semi-metal with several natural sources like minerals, rocks, soils and sediments formed from these arsenic-containing rocks, as well as geothermal and volcanic phenomena. Moreover, it is a chemical element used as a glass clarifier, in fireworks and in the pesticide industry. Arsenic has different chemical forms, including organic and inorganic species, with the later presenting higher toxicity [3][4][5][6].
Inorganic arsenic is considered a carcinogen, belonging to Group 1 of the International Agency for Research on Cancer (IARC), because long-term exposure is associated with an increased risk of several carcinomas, including skin, bladder, lung, kidney, liver and prostate [4]. Additionally, there is emerging evidence of causing skin lesions, neurotoxicity, cardiovascular diseases, diabetes and negative impacts on foetal and child development [7]. Human exposure to inorganic arsenic occurs mainly by the consumption of groundwater naturally containing high levels of inorganic arsenic, food prepared with this water, food crops irrigated with water sources with high arsenic content or that were treated with phosphate-based fertilizers and pesticides [6][8].
In areas where arsenic is naturally present in high levels, the foods that generally contribute mostly to daily intake are cereals and beans, namely rice [8]. Rice (Oryza sativa L.) feeds approximately 50% of the world population [9] and is one of the most cultivated and consumed cereals in the world [10]. According to the Food and Agriculture Organization (FAO), in 2018, around 517.5 million tons of rice were produced worldwide [11]. While Europe is not self-sufficient in rice production and is the third largest importer in the world, Portugal, along with Italy, Spain and Greece, is one of the European countries with the highest production and consumption of rice per capita [11][12][13]. Portugal produces 160,794 Tonnes of rice per year and the average consumption is 15.9 kg per person per year [14].
Rice is a widely used source of carbohydrates during weaning due to its availability, pleasant taste, nutritional value and relatively low allergenic potential. In addition, rice and derived products, such as starch, flour and syrup are used in different baby foods [15]. Rice is not consumed as harvested; it undergoes processing including several stages: Drying to reduce the moisture content of the paddy (harvested rice), cleaning of impurities, removing of the husk, milling to remove hulls and brans (for white rice) and separation of cracked rice (Figure S1, Supporting Information) [16].
The cultivation conditions and the plant’s morphology favour the absorption of arsenic and its accumulation in the grain [1]. The concentration varies according to the soil in which the rice is grown and the type of rice [15]. Numerous investigations have shown that rice grains in arsenic endemic areas contain more than 90% inorganic arsenic [17]. Most of the inorganic arsenic in rice is concentrated in the husk and bran, with concentrations 10 to 20 times higher than the rice grain [15]. Therefore, polished rice is expected to contain lower concentrations of arsenic than whole grains, since the outer layer of the rice was removed [18].
Other authors, worldwide, have reported average concentrations up to 350 µg kg−1 of inorganic arsenic [19], with Portuguese rice presenting averages up to 300.8 µg kg−1 [20]. Therefore, public health actions are needed to reduce human exposure to arsenic, particularly in areas with naturally high levels in groundwater [8].
In 2015, a regulation was established concerning the maximum levels of inorganic arsenic in foodstuffs, adding limits in rice and rice products. The limit for uncooked white rice is 0.20 mg kg−1, for stewed rice is 0.25 and 0.10 mg kg−1 for rice for the production of infant food and young children [21].
There are several spectroscopic analytical methodologies for determining metals that allow to extend the scale of concentration of elements to levels of ppm, ppb or even ppt, and perform multi-elemental analysis. The analytical methodologies reported for the determination of inorganic arsenic in rice are in decreasing limits of detection order: Atomic absorption spectrometry with flame (FAAS), with graphite chamber (GFAAS) and with hydride generation (HG-AAS), and inductively coupled plasma mass spectrometry (ICP-MS) [20][22][23]. Additionally, other techniques such as pulse differential voltammetry and square wave voltammetry, both used with the anodic stripping mode are also suitable for the determination of arsenic in rice [24].
2. Risk Assessment
The risk assessment was performed assuming arsenic concentrations in rice of 29.3 and 100 µg kg−1 (average and worst-case scenario, respectively); 19.1 and 25.1 kg (children and adults, respectively) as average annual rice consumption, 47.9 and 59 kg (children and adults, respectively) as 95th percentile of annual rice consumption; and an average weight of 24 and 69 kg (for children and adults, respectively). Using this data, it can be observed that the EDI surpassed the BMDL10 of 0.3 µg kg−1 of bw/day for children when considering the worst-case scenario contamination and the 95th percentile rice consumption (182.3%) (Figure 1).
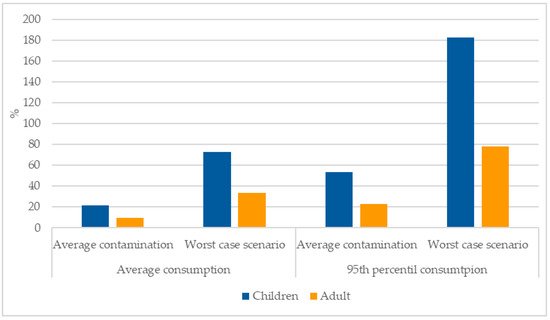
Figure 1. Percentage of the EDI (µg kg−1 bw/day) versus the BMDL10 (0.3 µg kg−1 bw/day).
Figure 1 shows that when using the worst-case scenario for the concentrations of arsenic found in rice the EDI approaches or surpasses the BMDL10, namely for children that have a higher consumption rate of rice per kg of body weight. When using average contamination concentrations, the higher value was 53.4% for children using the 95th percentile of rice consumption.
Using the average exposure to inorganic arsenic for children we can observe that, as expected, the percentage was higher for brown rice (34.2%) than for white rice (12.2%). Moreover, short grain had a higher percentage (22.9%) than short grain (17.8%). Regarding the regions, Tejo and Sado presented clearly higher percentages (37.1%) than Mondego (19.5%) and unknown origin (17.1%).
Observing the present results, there is low risk to consumers through the exclusive consumption of rice, considering that only using the scenario of 95th percentile of rice consumption and the highest contamination found, the children EDI surpassed the lowest BMDL. However, there are groups for which their exposure is higher, thus increasing the risk associated. This is the case for gluten intolerant people, celiacs, for whom the impossibility of consuming cereal-based foods entails rice as an alternative. Rice being the alternative to cereal-based pasta, its consumption is higher by celiacs than that by a non-celiac person. If it is assumed that the consumption of rice by celiacs is approximately double that of a non-celiac person the EDI will also duplicate.
Additionally, there are also other sources of inorganic arsenic in food (fish, molluscs, water) that should also be included for a more accurate risk assessment [4][7]. This can be observed in certain ethnic groups that have a daily exposure of inorganic arsenic in a diet of about 1 µg kg−1 bw/day, and also in high consumers of algae-based products that can have a dietary exposure to inorganic arsenic of about 4 µg kg−1 bw/day [7]. These products, namely seaweeds, are becoming part of the Western populations’ diet (consumption of sushi for example), particularly due to their health benefits and essential elements [25][26]. The increased seaweed consumption in the last few years highlights that the exposure to contaminants, namely metals and metalloids, through non-traditional foods is a growing reality that should be accounted for [27].
In 2009, EFSA estimated that national exposures to inorganic arsenic through food and water in 19 European countries ranged from 0.13 to 0.56 µg kg−1 bw/day for average consumers, and from 0.37 to 1.22 µg kg−1 bw/day for the 95% percentile, values that are higher than the BMDL10 (0.3 µg kg−1 bw/day) [7]. In 2014, EFSA estimated the average food exposure to inorganic arsenic among infants and the values ranged from 0.20 to 1.37 µg kg−1 bw/day. The food exposure average among the adult population (including adults, the elderly) ranged from 0.09 to 0.38 µg kg−1 bw/day, values that also surpassed the BMDL10. EFSA also confirmed that grains and cereals were the class of food that contributed the most for these values [28].
These values suggest that there are multiple sources of inorganic arsenic in food and water and that the BMDL10 value is easily surpassed by the EDI on several occasions. This highlights the importance of determining the inorganic arsenic in food and water, namely on grains and cereals, the main contributors for EDI of this contaminant.
3. Conclusions
Organic and inorganic arsenic is mainly present in water, originating from different sources, with foods that are irrigated with large amounts of water, such as rice, accounting for a greater exposure when compared to other cereals.
In this study, the identification and quantification of inorganic arsenic in rice was performed by ICP-MS. The methodology used proved to be adequate, allowing for an MDL of 3.3 µg kg−1 and a MQL of 10 µg kg−1, while the accuracy of the method ranged between 89% and 114%.
The results obtained showed that of all the analysed samples contained inorganic arsenic, however, none above what is stipulated by law for inorganic arsenic present in rice. It is also concluded that the brown rice samples are the ones that present the highest average of inorganic arsenic (47.1 µg kg−1). Short rice had higher average concentration (31.5 µg kg−1) than long rice (24.5 µg kg−1). Among the different regions of origin in Portugal Tejo and Sado region presented the highest amount of inorganic arsenic (51.1 µg kg−1).
Considering the risk assessment carried out, it can be concluded that only in very specific cases (children in the 95th percentile of rice consumption and worst-case scenario concentration) the BMDL10 (0.3 µg kg−1 of bw/day) is surpassed by the EDI.
It should be noted that rice is not the only source of inorganic arsenic. Therefore, other sources that also contribute to the daily intake should also be considered for a correct risk assessment. Additionally, there are population groups that present a higher risk to the exposure of inorganic arsenic like children, celiac people, some ethnic groups and high consumers of algae-based products that can highly exceed the BMDL10.
This entry is adapted from the peer-reviewed paper 10.3390/foods11030277
References
- Ferreiro, M.; Antunes, H. Segurança alimentar e sustentabilidade: O caso do setor do arroz no Vale do Tejo e Sorraia: Perceção e práticas. Master’s Thesis, University Institue of Lisbon, Lisbon, Portugal, 2017.
- Cardoso, M. Segurança alimentar, ajuda pública ao desenvolvimento e pobreza. In Proceedings of the 7th Congress of African Studies, Lisboa, Portugal, 9–11 September 2010. CIEA7 #22.
- Souza, J.M.O.; Carneiro, M.F.H.; Carolina, A.; Paulelli, C.; Grotto, D.; Magalhães, A.M. Arsénio e arroz: Toxicidade, metabolismo e segurança alimentar. Quim. Nova 2014, 38, 118–127.
- International Agency for Research on Cancer(IARC) Arsenic and Arsenic Compounds Monograph. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 41–93.
- Andrade, D.; Rocha, M. A toxicidade do arsenio e a sua natureza. Rev. Acadêmica Oswaldo Cruz 2016, 3, 102–111.
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201.
- EFSA. Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351.
- WHO. Exposure to Arsenic: A Major Public Health Concern. Agriculture 2010, 79, 90–100.
- de Oliveira, R.M.; Antunes, A.C.N.; Vieira, M.A.; Medina, A.L.; Ribeiro, A.S. Evaluation of sample preparation methods for the determination of As, Cd, Pb, and Se in rice samples by GF AAS. Microchem. J. 2016, 124, 402–409.
- Garcia, I. Influência de Diferentes Variedades de Arroz Carolino no Seu Comportamento em Cozedura. Ph.D. Thesis, Istituto Politécnico de Coimbra, Coimbra, Portugal, 2017.
- FAO. Nations–Crops. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 3 November 2021).
- De Jesus, M.G. Caracterização das Diferentes Designações Comerciais de Arroz. Master’s Thesis, University of Oporto, Oporto, Portugal, 2019.
- Coelho, I.; Gueifão, S.; Pinto, T.; Castanheira, I. Estudos de especiação de arsénio em arroz. Obs. Epidemiológico 2015, 2, 6–7.
- Instituto Nacional de Estatiscica, I. Estatísticas Agrícolas 2018. 2019. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=320461359&PUBLICACOESmodo=2 (accessed on 21 December 2021).
- Hojsak, I.; Braegger, C.; Bronsky, J.; Campoy, C.; Colomb, V.; Decsi, T.; Domellöf, M.; Fewtrell, M.; Mis, N.F.; Mihatsch, W.; et al. Arsenic in rice: A cause for concern. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 142–145.
- Fernandes, R.M. Efeito da temperatura e humidade no rendimento industrial de arroz das variedades Ariete, Euro, Gládio e Sírio. Master’s Thesis, Instituto Superior de Agronomia University of Lisbon, Lisbon, Portugal, 2015.
- Kumarathilaka, P.; Seneweera, S.; Ok, Y.S.; Meharg, A.; Bundschuh, J. Arsenic in cooked rice foods: Assessing health risks and mitigation options. Environ. Int. 2019, 127, 584–591.
- Suriyagoda, L.D.B.; Dittert, K.; Lambers, H. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric. Ecosyst. Environ. 2018, 253, 23–37.
- Torres-Escribano, S.; Leal, M.; Vélez, D.; Montoro, R. Total and Inorganic Arsenic Concentrations in Rice Sold in Spain, Effect of Cooking, and Risk Assessments. Environ. Sci. Technol. 2008, 42, 3867–3872.
- Simões, A.C.P. Avaliação da Presença de Arsénio em Arroz e Produtos Derivados de Arroz. Master’s Thesis, Instituto Superior de Agronomia University of Lisbon, Lisbon, Portugal, 2014.
- Comissão Europeia Regulamento (UE) 2015/1006 da Comissão de 25 de junho de 2015 que altera o Regulamento (CE) no 1881/2006 no que diz respeito aos teores máximos de arsénio na forma inorgânica nos géneros alimentícios. J. Of. da União Eur. 2015, 2015, 1993–1995.
- Pétursdóttir, Á.H.; Friedrich, N.; Musil, S.; Raab, A.; Gunnlaugsdóttir, H.; Krupp, E.M.; Feldmann, J. Hydride generation ICP-MS as a simple method for determination of inorganic arsenic in rice for routine biomonitoring. Anal. Methods 2014, 6, 5392–5396.
- Ashmore, E.; Molyneux, S.; Watson, S.; Miles, G.; Pearson, A. Inorganic arsenic in rice and rice products in New Zealand and Australia. Food Addit. Contam. Part B Surveill. 2019, 12, 275–279.
- Pungjunun, K.; Chaiyo, S.; Jantrahong, I.; Nantaphol, S.; Siangproh, W.; Chailapakul, O. Anodic stripping voltammetric determination of total arsenic using a gold nanoparticle-modified boron-doped diamond electrode on a paper-based device. Microchim. Acta 2018, 185, 324.
- Panebianco, F.; Nava, V.; Giarratana, F.; Gervasi, T.; Cicero, N. Assessment of heavy- and semi-metals contamination in edible seaweed and dried fish sold in ethnic food stores on the Italian market. J. Food Compos. Anal. 2021, 104, 104150.
- Mac Monagail, M.; Morrison, L. Arsenic Speciation in a Variety of Seaweeds and Associated Food Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–310.
- Filippini, M.; Baldisserotto, A.; Menotta, S.; Fedrizzi, G.; Rubini, S.; Gigliotti, D.; Valpiani, G.; Buzzi, R.; Manfredini, S.; Vertuani, S. Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere 2021, 263, 127983.
- EFSA. Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014, 12, 3597.
This entry is offline, you can click here to edit this entry!
