Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
The food packaging sector generates large volumes of plastic waste due to the high demand for packaged products with a short shelf-life. Biopolymers such as starch-based materials are a promising alternative to non-renewable resins, offering a sustainable and environmentally friendly food packaging alternative for single-use products.
- starch-based materials
- processing
- food packaging
1. Advance in Preparation of Functional Starch-Based Food Packaging
Recent advances in the development of starch-based materials for food packaging applications are based on the combination of the emergence of new preparation techniques, equipment and the manipulation of matter at the nanometer scale, which have allowed us to achieve special properties with adequate performance [1][2].
1.1. Incorporating Bioactive
In recent years the incorporation of additives such as antioxidants and antimicrobials in starch-based films plays a key role in improving functional properties. These active packaging films provide a semi-permeable barrier that helps to extend shelf life by reducing the migration of moisture, loss of solutes from fruit respiration and oxidation reaction [1][3]. Essential oils are a clear example of active components in food packaging, their antioxidant and antimicrobial properties have improved the quality and safety of food [4]. Essential oils are natural substances composed of alcohols, phenols, terpenes, esters and among other bioactive agents, whose main function is the release of their active components avoiding microbial and fungal attack and oxidation of food [1]. Oregano oil, thyme [5], cinnamon bark, clove [6], ho wood (Cinnamomum camphora), and cardamom [7] have been evaluated as additives in the control of various pathogens.
Raigong et al. [8] evaluated the addition of clove oil and cinnamon oil in starch films against S. aureus, C jejuni and E. coli, and the results showed the inhibition of the pathogens. Clove oil inhibited between 22–100%, while cinnamon oil was effective against C. jejuni (19–22% inhibition) and E. coli (33–40% inhibition), respectively. Souza et al. [7] evaluated that the addition of Pickering emulsions to essential oils, ho wood (Cinnamomum camphora), cardamom, and cinnamon, showed that the ho wood oil lowered water vapor transmission rate, improving the release of the active compound. Ho wood oil was the most promising with regard to being applied as a biodegradable active packaging. The addition of natural extracts from various sources, such as fruit by-products, has been evaluated in starch-based films. Mango puree and pineapple pomace were incorporated into maize starch-gelatin films, improving the physicochemical properties of the films and increasing the antioxidant activity and antimicrobial activity [1]. Table 1 lists the most recent studies on starch-based bioactive systems prepared by film casting method for food packaging applications.
Table 1. Bioactive system, processing techniques and main results of bioactive starch based materials for food applications.
| System | Starch Source | Bioactive | Results | Application | Reference |
|---|---|---|---|---|---|
| Poly (vinyl alcohol)-corn starch | Corn | Pineapple peel extract as a natural antioxidant agent | Film thickness and water vapor permeability increased slightly, antioxidant capacity increased. | Food Packaging | [9] |
| Lemon essential oil/surfactants (Span 80, Tween 80)/corn and wheat starch | Corn and wheat | Lemon essential oil | All concentrations of lemon oil were effective against selected bacteria (both Gram-negative and Gram-positive) compared with control film (without lemon oil) | Food Packaging | [10] |
| Chitosan-Starch-antioxidants | Rice | Antioxidants (from cranberry, blueberry, beetroot, pomegranate, oregano, pitaya and resveratrol, thymol and carvacrol) | The addition of natural extracts gives chitosan-starch a higher apparent density values. The addition of natural extracts provided chitosan-starch films with better thermal and physical properties | Food Packaging | [11] |
| Sodium alginate-starch | Yucca | Anthocyanin and betanin (from the exocarp of the black eggplant (Solanum melongena) and the mesocarp of beet (Beta vulgaris)) | Incorporation of natural extracts influenced the mechanical properties, however did not influence film thickness or water vapor permeability. Films with eggplant extract had higher antioxidant activity against the (DPPH) radical and were more sensitive to the exposure of gaseous amines in comparisonwith films with beet extract. | Food Packaging | [12] |
| Mung bean starch-chitosan (MSC) Water chestnut starch-chitosan (WSC) | Mung bean/Water chestnut | Hydrophobic perilla oil | The results showed that the cheese coated by WSC film containing perilla oil presented better treatment performance in terms of microbial growth delay, weight loss and shelf life length. | Food Packaging for cheese | [13] |
| Cassava starch- essential oil-sodium bentonite nanclay | Cassava | Cinnamon essential oil | The meatballs stored at ambient temperature in cassava starch film incorporated with cinnamon oil and nano-clay, significantly inhibited the microbial growth till 96 h below the FDA limits (106 CFU/g) in foods compared to control films that exceeded the limit within 48 h. | Food Packaging for meatballs | [14] |
| Starch-furcellaran-lavender essential oil-gelatin | Potato | Lavender essential oil | Antioxidant properties proved to be significantly enhanced with increasing lavender essential oil concentration. The solubility, water absorption and degree of swelling of the film decreased with increasing concentration of oils. | Food Packaging | [15] |
| Tapioca starch-cinnamon bark essential oil-glycerol | Tapioca | Cinnamon bark essential oil | Increasing cinnamon bark essential oil improves tensile strength and antibacterial activity of the film and preserved the freshness of the beef during 15 days of storage. | Food Packaging for fresh beef | [16] |
| (Gelatin-pectin-starch)-(gelatin-pectin)-(gelatin-starch)-(starch-pectin) | Potato | Mentha pulegium and Lavandula angustifolia essential oils | The incorporation of essential oils resulted in films with enhanced antibacterial properties, lower water vapor permeability, and reduced mechanical properties | Food Packaging | [17] |
| Carvacrol essential oil-corn starch-montmorillonite-tween 80/Carvacrol essential oil-glycerol-corn starch | Corn | Carvacrol essential oil | The starch-montmorillonite-carvacrol essential oil hybrid films showed antimicrobial behavior against E. coli. | Food Packaging | [18] |
| Arrowroot starch-carnauba wax nanoemulsion-cellulose nanocrystals-essential oils from Mentha spicata and Cymbopogon martinii | Arrowroot | Mentha spicata and Cymbopogon martinii | The essential oils from Mentha spicata and Cymbopogon martinii incorporation improved the thermal stability of the films and provided excellent protection against fungi Rhizopus stolonifer and Botrytis cinerea. | Food Packaging | [19] |
| Corn starch-thyme essential oil microcapsules | Corn | Thyme | The addition of thyme essential oil microcapsules to starch films increased the opacity, thickness, tensile strength and water solubility. They also showed an inhibitory effect against Botryodiplodia theobromae Pat and Colletotrichum gloeosporioides Penz and extended the shelf life of mangoes up to 10 days at 25 °C. | Food Packaging for mango | [20] |
| Corn starch-PVA- neem and oregano essential oils | Pea | Neem and oregano | Starch-PVA films with 6.7% of oregano essential oils exhibited the best physical properties, without significant differences with respect to the starch-PVA matrix, while exhibiting antibacterial activity. | Food Packaging | [21] |
1.2. Starch Nanostructures (SNEs)
The field of natural biopolymers have shown great potential for important, rapidly growing applications ranging from green electronics, food packaging, dye and heavy metal removal, oil/water separation, therapeutic agent delivery, tissue engineering scaffolds, biological devices, optics, and sensing [22]. However, the application of advanced functional biopolymer materials suffers from their poor processability and weak mechanical properties. Regarding this, there are enormous challenges to break the strong intermolecular interactions (hydrogen bonding) in their native forms, while re-establishing predominant hydrogen bonding in the processed materials in a cost-effective way. The introduction of one or more new functional groups into native polysaccharides alters their physical, chemical and, above all, biological properties. The biological properties of polysaccharide derivatives depend on the molecular weight, the type of modification, the type of native polysaccharide, the conditions of the modification process, the solubility and the conformation of the polysaccharide. The manipulation of matter at the nanometer scale (1 to 100 nm) has recently been studied to create new materials and devices with special properties and adequate performance. The unique properties of nanoparticles depend on the size and shape, charge and surface modification, and hydrophobicity of the starting material [23]. For example, starch nanoparticles prepared by acid hydrolysis from waxy corn and high amylose maize starch exhibit a crystal structure and size of type A-type, B-Type, and 50 nm, 540 nm, respectively, while their morphology was polygonal and smaller starch granules, pores, respectively [24].
In order to alter the crystalline structure of the starch, SNEs are obtained mainly by top-down and bottom-up methods. In the “top-down” method, macroscopic materials are reduced from the microscale to the nanoscale through physicochemical processes such as acid hydrolysis [25][26] due to the sensitivity of the amorphous rings in starch granules to acid treatment, homogenization [27], crushing [28][29], gamma irradiation [30], and ultrasound [31][32]. Acid hydrolysis and ultrasonication methods are particularly effective in breaking up the aggregates of nanoparticles formed through hydrogen bonds, thereby reducing the size and polydispersity of nanoparticles [33].
In the “bottom-up” process, SNEs can be obtained from a buildup of starch molecules in a controlled manner that is regulated by thermodynamic means such as regeneration [34] nanoprecipitation or self-assembly [28]. Micro-nano emulsion and nanoprecipitation are very simple and convenient methods for producing nanoparticles with a desired size [35]. For example, starch granules are dispersed in water or dimethylform sulfoxide, completely gelatinized at 100 °C, and then precipitated by dropwise addition of nonsolvents (such as methanol, ethanol, isopropanol, n-propanol) to obtain SNPs with different sizes [36][37]. In addition, it has been shown that the combination between chemical methods, for example acid hydrolysis, and physical methods such as ultrasonication, generates higher homogeneity and yield in the obtained starch nanostructures [34][38][39].
Among the recently studied starch-based nanostructures are nanoparticles [40][41], nanospheres [42][43], nanocrystals [39][44][45], nanomicelles [46][47], nanogels [48] and nanofibers [49]. Table 2 summarizes the main preparation methods and size of starch nanostructures. Emphasis is placed on their responsiveness, permeability, toxicity, interactions with other components and applications. The aim of producing such nanocrystals or nanoparticles is to use them as fillers in polymeric matrices to improve their mechanical and/or barrier properties. Starch nanoparticles are non-toxic and respond to pH, temperature, light and other stimuli. Starch nanoparticles have a wide range of applications, such as improving the mechanical properties of films and gels, stabilizing emulsions, use as a fluorescent indicator, forming or directing agent in self-assembling structures, scaffolds, and reconstruction of hollow organs.
Table 2. Preparation methods and size of starch nanostructures.
| Nanoestructure | Raw Materials | Preparation Method | Size (nm) | Reference |
|---|---|---|---|---|
| Nanocrystal | Potato | Acid hydrolysis-ultrasonication | 40–70 | [50] |
| Nanocrystal | Pea | Acid hydrolysis-ultrasonication | 30–80 | [39] |
| Nanocrystal | Waxy | Acid hydrolysis-ultrasonication | 70–100 | [51] |
| Nanocrystal | High amylose maize | Acid hydrolysis | 118–130 | [42] |
| Nanospheres | Soluble starch | Micro-emulsion | 50–350 | [52] |
| Nanospheres | Native sago starch | Nanoprecipitation | 270–420 | [43] |
| Nanospheres | Corn | Microemulsion | 96–100 | [53] |
| Nanospheres | Corn | Nanoprecipitation | 90–100 | [54] |
| Nanospheres | Potato | Acid hydrolysis-ultrasonication | 40 | [55] |
| Nanogels | Corn, potato, and pea starch | Reverse emulsification | 100 | [56] |
| Nanogels | α-starch | Chemical crosslinking | 30 | [48] |
| Nanogels | Starch/poly(alginic acid-cl-acrylamide) | Chemical crosslinking | 380 | [57] |
| Nanogels | CMS | EB radiation N | 380 | [58] |
| Nanogels | Potato | Chemical crosslinking | 120–160 | [59] |
| Nanofibers | Corn | Electrospinning | 750–900 | [60] |
| Nanofibers | High amylose Maize starch |
Cross-linking/Electrospinning | 300–700 | [61] |
| Nanofibers | Corn | Coaxial Electrospinning | 110–160 | [62] |
| Nanofibers | High-amylose maize starch and nGO | Electrospinning | 30–50 | [63] |
| Nanofibers | Soluble starch | Coaxial electrospinning | 90–250 | [49] |
| Micelle | Corn | Graft copolymerization/self-assemble | 20–30 | [64] |
| Micelle | Waxy Maize | Emulsion/self-assemble | 60–70 | [65] |
| Micelle | Soluble | Schiff-base bonds | [66] | |
| Micelle | Starch-octanoic | Graft copolymerization/self-assemble | 400–600 | [67] |
| Nanoparticulas | Waxy Maize | Acid hydrolysis-ultrasonication | 50–80 | [38] |
| Nanoparticulas | Waxy Maize | Enzymatically hydrolyzed-emulsion cross-linking | 80–130 | [34] |
| Nanoparticulas | Pea | Precipitation-complex formation | 50–100 | [40] |
| Nanoparticulas | Corn | Complex formation | 10–20 | [34] |
2. Starch Based Materials Application in Food Industry
A fundamental part in the food packaging industry is to innovate, develop new materials with improved properties, reduce food waste, and be economically viable and sustainable, while at the same time complying with the standards of quality, safety and functionality; contain, protect and conserve. Another important issue is that developed materials must facilitate product handling, although they must also preserve nutritional value. Currently, the use of bioplastics has increased significantly, and it is estimated that by 2022 the production of bioplastics will be around 2.44 million tons due to a great demand for biopolymers for various applications and product [68]. This increase in the manufacture of biopolymers as substitutes for conventional packaging is mainly due to the biodegradability, biocompatibility and low cost of these starch base materials [69] as starch by itself or in combination with other biopolymers, have been used in the preservation of fresh products.
One of the strategies to generate interest among consumers and for the packaging industry to commercialize starch-based materials have been the development of smart and/or functional materials to extend the shelf life of packaged foods (Figure 1). In this context, the main work has focused on: (i) improving the materials so they provide a better barrier to oxygen and water vapor materials by mixing different materials and incorporating micro/nano structures; and (ii) adding bioactive substances such as antioxidant or antibacterial agents by means of micro/nano encapsulation [70].
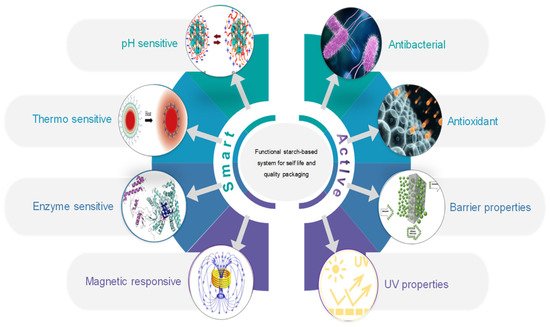
Figure 1. Active and smart starch-based food packaging to improve shelf life.
Active packaging deliberately modifies the product or environment to improve food safety and quality. Hence, increased attention has been paid to the preparation of bioactive and smart packaging films by active films having antibacterial, antioxidant, and barrier properties [71][72]. Other examples of active packaging include oxygen scavengers to decrease fat oxidation, ethylene scavengers to minimize fruit and vegetable ripening, humidity and odor absorbers [73][74][75][76][77].
Smart packaging informs the consumer about kinetic changes related to the quality of the food or the environment it contains, to minimize losses and ensure food quality. Hence, the temperature can be monitored, providing a thermal history of the foods storage, and informing the most suitable consumption conditions. Some studies have been carried out to produce such materials, and commercial packaging is available in the market. Jederman et al. [78] monitored the temperature curves allowing the evaluation of the cooling efficiency in bananas, the effect of changes in packaging and the respiration heat. Commercial brands are patented by MonitorMark™ and commercialized as a Time–Temperature Indicators (TTI) sensor developed by 3M™ (3M™, Maplewood, MN, USA) and CoolVu indicator developed in Freshpoint-Switzerland.
Fish and meat products are highly susceptible to decomposition by oxidation of fats showing color changes, (e.g., discoloration of pigments such as myoglobin, carotenoids), and off-odors and flavors (e.g., rancidity as a result of lipid oxidation), which leads to nutrient losses (e.g., oxidation of vitamin E, β-carotene, ascorbic acid) and adversely affects the quality [79]. In order to prevent those problems, vacuum packaging has been used as this method does not always remove all oxygen under the packaging. That is why it has been necessary to develop novel packaging materials to prevent oxidation and meat quality loss. In this sense, oxygen scavengers have been supplied in packaging to eliminate the residual oxygen. The oxygen scavengers are incorporated in sachets, films or labels to prevent food products contamination or accidental consumption. Among the substances incorporated are iron powder, ascorbic acid, dyes, enzymes (glucose oxidase and alcohol oxidase), unsaturated fatty acids (oleic or linoleic acids), and immobilized yeast [80]. On the other hand, thermoplastic starch films have also been developed with the purpose of reducing lipid oxidation in foods. Panrong et al. [81] prepared thermoplastic starch films incorporating low-density polyethylene and green tea. They proved that the hydrophobicity of films allows a reduction in the lipid oxidation of packaged soybean oil, which was effectively reduced by up to 38% depending on the TPS ratio used. Similarly, Piñeros-Hernandez et al., [82] prepared edible cassava starch films carrying rosemary antioxidant extracts for use as potential active food packaging. They reported the films enhanced the UV-blocking properties of the films.
Smart packaging films based on a change of color have been developed, and they have pH-sensitive and responsive indicators (e.g., anthocyanins, betacyanins, and curcumin) in a biopolymer-based matrix [71]. These materials are usually synergistically blended with other polymers (PVA, PLA, carrageenan, chitosan), can also respond to magnetic field, or have enzyme-responsive characteristics [72]. Recently, the application of natural pigments and polymer carriers has shown great potential in smart packaging based on pH-responsive indicators [83]. A research work conducted by Silva-Pereira et al. [84] revealed the use of blueberry residues as a potential visual pH indicator in the monitoring of fish spoilage. The indicator carrier matrix was corn starch and chitosan and showed good pH sensitivity and thermal stability. Similarly, films based on cassava starch and anthocyanins showed high pH sensitivity over a wide pH range, which allows monitoring of the quality of various foods [85]. In addition, potato starch-based films with anthocyanins can successfully display the color difference at pH 1–12 and detect the fresh stage (pH = 5.8) and spoiled stage (pH = 8) of pork, demonstrating the potential of potato starch for food product quality detection [86]. Shapi’i et al. [87] evaluated the effect of incorporating chitosan nanoparticles into a starch matrix on the antibacterial properties of the film. The authors found that the starch/chitosan nanoparticle film used to package cherry tomatoes effectively inhibited the growth of microorganisms (7 × 102 CFU/g) compared to pure starch film (2.15 × 103 CFU/g). Another way to inhibit the growth of microorganisms in starch films was reported by Diaz-Galindo et al. [88] by adding a cinnamon oil emulsion to the matrix that reduced the growth rate of Botrytis cinerea by 66%, preventing further contamination of the fruit during storage and transport.
The incorporation of anthocyanin-rich bay laurel berry extracts (BBE) into tapioca starch to develop food packaging films with antioxidant and pH-sensitive properties was studied by Yun et al. [89]. The work demonstrated a significant increase in the DPPH radical scavenging ability of the composite film (24.39–75.01% under 5 mg mL−1) with the incorporation of BBE into the starch matrix. It was observed that when the starch-BBE film was exposed to hydrogen chloride, the color of the film changed from purple to red. The film quickly turned blue and then olive when exposed to ammonia gas. Jayakumar et al. [90] incorporated nutmeg oil, ZnO NP and ham extract into starch/PVA based films. These films showed pH sensitive and antibacterial properties. Under acidic pH, the dark purple extract turned cherry red, while at alkaline pH it changed to brownish yellow to light green at neutral pH. The film mixed with ZnO NP and nutmeg oil inhibited the growth of the foodborne pathogen Salmonella typhimurium. Similarly, the results of Mustafa et al. [91] demonstrated a variation in the coloration of smart and bioactive PVA/starch/propolis/procyanidin rosemary extract films depending on pH; reddish to blue under acidic pH, blue under neutral pH and yellow under alkaline pH. The maximum diameters of the film inhibition zone against E. coli and methicillin-resistant S. aureus were 21 and 15 mm, respectively. Table 3 summarizes some applications in the packaging of various foods, such as fruits and vegetables, bakery goods, meat, and starch-based materials indicating good prospects for commercial utilization.
Table 3. Packaging system, food application and mains results of pre-commercial studies of starch based materials.
| Packaging System | Processing Techniques | Function | Food Application | Results | References |
|---|---|---|---|---|---|
| Rice starch in combination with chitosan, emulsifier (sodium caseinate), and red palm oil. | Dipping | Enhancing the shelf life of walnuts | To coat dried walnut kernels | Films with higher in elongation at break, but lower in tensile strength. Film is more flexible than the other corn and wheat starch films tested in this study. Rice starch with high flexibility produces a uniform layer on the surface of walnut. | [92] |
| Cassava starch at different concentrations (1%, 2%, 3% and 4%) | Dipping | Delay the ripening of papaya fruit (Carica papaya) | Coating papaya fruit (Carica papaya) | All cassava starch coating concentrations reduced fruit maturation and anthracnose, with the 2%, 3% and 4% coatings giving 100% disease control. | [93] |
| Nano-SiO2-potato starch | Film | Preservation the white mushroom | White mushroom | The water resistance and mechanical properties of the films were improved with the addition of nano-SiO2. Resistance to ultraviolet and thermal aging was also improved. Finally, they were more efficient against Escherichia coli (E. coli) than Staphylococcus aureus (S. aureus), improving the preservation of white fungi. | [94] |
| Corn starch (TPS) and chitosan oligomers | Film | Package perishable foods such as strawberries, ricotta, and flavored breads, | Strawberries, ricotta, and flavored breads. | Sachet type packages demonstrated to have a notable antimicrobial capability against molds and yeasts. Flavored breads were the least susceptible product to the microbial development, while strawberries and ricotta presented the highest molds and yeasts growth, respectively. | [95] |
| Yam starch-glycerol | Film | Extend storage life of strawberries stored at 4 °C and 85% RH | Strawberries | Yam Starch films significantly reduced decay of the fruits compared to control and extended the shelf life of strawberries by 21 days. | [96] |
Thus, Oliveira et al. [93] evaluated different concentrations of cassava starch in the protection of papaya fruit, reducing the ripening of the fruit and controlling diseases by 100%. Castillo et al. [95] made a Sachet type package of corn starch and chitosan oligomers for perishable foods such as strawberries, ricotta, and flavored breads. Table 4 lists the starch-based products currently available and marketed for food packaging applications where Biotec, Novamont and BioBag Americas are the main manufacturing companies.
Table 4. Commercially available starch-based materials for food packaging applications.
| Material | Product | Manufacturing Company | Web Site |
|---|---|---|---|
| Granules based on corn powder/polyester + corn powder | Bio Degradable Bio One and Bio Base Rangdaneh Sirjan | RANGDANEH SIRJAN Co. Sirjan-IRAN | http://www.rangdaneh.ir (accessed on 20 November 2021) |
| BIOTEC contains 75% renewable feedstock and has a 69% biobased carbon share according to ASTM D6866 and ISO 16620-2. | BIOPLAST 105 BIOPLAST 300 BIOPLAST 400 BIOPLAST 500 BIOPLAST 900 BIOPLAST GF 106/02 BIOPLAST GS 2189 |
BIOTEC GmbH and Co. KG Emmerich am Rhein-Alemania |
https://es.biotec.de (accessed on 20 November 2021) |
| Starch | Mater-Bi | Novamont, S.L.U. Novara-Italia |
https://www.novamontiberia.es/ (accessed on 20 November 2021) |
| Starch-PBAT | BioAgri Mulch Film | BioBag Americas, Inc. Palm Harbor-Canadian |
https://www.biobagusa.com (accessed on 20 November 2021) |
| Starch from the potato processing industry and/or grain, root or seed flour based resources | Solanyl® | Rodenburg Oosterhout- The Netherlands |
https://biopolymers.nl (accessed on 20 November 2021) |
This entry is adapted from the peer-reviewed paper 10.3390/polysaccharides3010007
References
- Susmitha, A.; Sasikumar, K.; Rajan, D.; Padmakumar, M.A.; Nampoothiri, K.M. Development and characterization of corn starch-gelatin based edible films incorporated with mango and pineapple for active packaging. Food Biosci. 2021, 41, 100977.
- Meng, L.; Xie, F.; Zhang, B.; Wang, D.K.; Yu, L. Natural biopolymer alloys with superior mechanical properties. ACS Sustain. Chem. Eng. 2018, 7, 2792–2802.
- Yao, X.; Qin, Y.; Zhang, M.; Zhang, J.; Qian, C.; Liu, J. Development of active and smart packaging films based on starch, polyvinyl alcohol and betacyanins from different plant sources. Int. J. Biol. Macromol. 2021, 183, 358–368.
- Vianna, T.C.; Marinho, C.O.; Marangoni Junior, L.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819.
- Cruz-Tirado, J.P.; Barros Ferreira, R.S.; Lizárraga, E.; Tapia-Blácido, D.R.; Silva, N.C.C.; Angelats-Silva, L.; Siche, R. Bioactive Andean sweet potato starch-based foam incorporated with oregano or thyme essential oil. Food Packag. Shelf Life 2020, 23, 100457.
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240.
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-based films enriched with nanocellulose-stabilized Pickering emulsions containing different essential oils for possible applications in food packaging. Food Packag. Shelf Life 2021, 27, 100615.
- Raigond, P.; Sood, A.; Kalia, A.; Joshi, A.; Kaundal, B.; Raigond, B.; Dutt, S.; Singh, B.; Chakrabarti, S.K. Antimicrobial Activity of Potato Starch-Based Active Biodegradable Nanocomposite Films. Potato Res. 2019, 62, 69–83.
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple peel extract incorporated poly(vinyl alcohol)-corn starch film for active food packaging: Preparation, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 187, 223–231.
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309.
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties. Materials 2018, 11, 120.
- Torres Vargas, O.L.; Galeano Loaiza, Y.V.; González, M.L. Effect of incorporating extracts from natural pigments in alginate/starch films. J. Mater. Res. Technol. 2021, 13, 2239–2250.
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of edible starch–chitosan film and its application in the storage of Mongolian cheese. Int. J. Biol. Macromol. 2013, 57, 17–21.
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J. Food Sci. Technol. 2018, 55, 1953–1959.
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the physical properties, antioxidant and antimicrobial activity of ternary potato starch-furcellaran-gelatin films incorporated with lavender essential oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101.
- Utami, R.; Khasanah, L.U.; Manuhara, G.J.; Ayuningrum, Z.K. Effects of Cinnamon Bark Essential Oil (Cinnamomum burmannii) on Characteristics of Edible Film and Quality of Fresh Beef. Pertanika J. Trop. Agric. Sci. 2019, 42, 1173–1184.
- Aitboulahsen, M.; El Galiou, O.; Laglaoui, A.; Bakkali, M.; Hassani Zerrouk, M. Effect of plasticizer type and essential oils on mechanical, physicochemical, and antimicrobial characteristics of gelatin, starch, and pectin-based films. J. Food Processing Preserv. 2020, 44, e14480.
- De Souza, A.G.; Dos Santos, N.M.A.; da Silva Torin, R.F.; Dos Santos Rosa, D. Synergic antimicrobial properties of Carvacrol essential oil and montmorillonite in biodegradable starch films. Int. J. Biol. Macromol. 2020, 164, 1737–1747.
- De Oliveira Filho, J.G.; Albiero, B.R.; Cipriano, L.; de Oliveira Nobre Bezerra, C.C.; Oldoni, F.C.A.; Egea, M.B.; de Azeredo, H.M.C.; Ferreira, M.D. Arrowroot starch-based films incorporated with a carnauba wax nanoemulsion, cellulose nanocrystals, and essential oils: A new functional material for food packaging applications. Cellulose 2021, 28, 6499–6511.
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of starch film containing thyme essential oil microcapsules on physicochemical activity of mango. LWT 2020, 131, 109700.
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antimicrobial Properties of Starch-PVA Blend Films as Affected by the Incorporation of Natural Antimicrobial Agents. Foods 2016, 5, 3.
- Meng, Q.; Liu, Z.; Han, S.; Xu, L.; Araby, S.; Cai, R.; Zhao, Y.; Lu, S.; Liu, T. A facile approach to fabricate highly sensitive, flexible strain sensor based on elastomeric/graphene platelet composite film. J. Mater. Sci. 2019, 54, 10856–10870.
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18.
- Yu, M.; Ji, N.; Wang, Y.; Dai, L.; Xiong, L.; Sun, Q. Starch-based nanoparticles: Stimuli responsiveness, toxicity, and interactions with food components. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1075–1100.
- Putro, J.N.; Ismadji, S.; Gunarto, C.; Soetaredjo, F.E.; Ju, Y.H. A study of anionic, cationic, and nonionic surfactants modified starch nanoparticles for hydrophobic drug loading and release. J. Mol. Liq. 2020, 298, 112034.
- Dufresne, A.; Kellerhals, M.B.; Witholt, B. Transcrystallization in Mcl-PHAs/cellulose whiskers composites. Macromolecules 1999, 32, 7396–7401.
- Liu, D.; Wu, Q.; Chen, H.; Chang, P.R. Transitional properties of starch colloid with particle size reduction from micro-to nanometer. J. Colloid Interface Sci. 2009, 339, 117–124.
- Lin, H.; Qin, L.Z.; Hong, H.; Li, Q. Preparation of starch nanoparticles via high-energy ball milling. J. Nano Res. 2016, 40, 174–179.
- Chen, C.-J.; Shen, Y.-C.; Yeh, A.-I. Physico-chemical characteristics of media-milled corn starch. J. Agric. Food Chem. 2010, 58, 9083–9091.
- Lamanna, M.; Morales, N.J.; García, N.L.; Goyanes, S. Development and characterization of starch nanoparticles by gamma radiation: Potential application as starch matrix filler. Carbohydr. Polym. 2013, 97, 90–97.
- Zhu, J.; Li, L.; Chen, L.; Li, X. Study on supramolecular structural changes of ultrasonic treated potato starch granules. Food Hydrocoll. 2012, 29, 116–122.
- Boufi, S.; Haaj, S.B.; Magnin, A.; Pignon, F.; Impéror-Clerc, M.; Mortha, G. Ultrasonic assisted production of starch nanoparticles: Structural characterization and mechanism of disintegration. Ultrason. Sonochem. 2018, 41, 327–336.
- Grieser, F.; Ashokkumar, M.; Sostaric, J.Z. Sonochemistry and sonoluminescence in colloidal systems. In Sonochemistry and Sonoluminescence; Springer: Berlin/Heidelberg, Germany, 1999; pp. 345–362.
- Kim, J.-Y.; Lim, S.-T. Preparation of nano-sized starch particles by complex formation with n-butanol. Carbohydr. Polym. 2009, 76, 110–116.
- Chin, S.F.; Azman, A.; Pang, S.C. Size controlled synthesis of starch nanoparticles by a microemulsion method. J. Nanomater. 2014, 2014, 9.
- Dong, Y.; Chang, Y.; Wang, Q.; Tong, J.; Zhou, J. Effect of operating conditions on size and morphology of amylose nanoparticles prepared by precipitation. Starch-Stärke 2015, 67, 365–372.
- Qin, Y.; Liu, C.; Jiang, S.; Xiong, L.; Sun, Q. Characterization of starch nanoparticles prepared by nanoprecipitation: Influence of amylose content and starch type. Ind. Crops Prod. 2016, 87, 182–190.
- Kim, H.-Y.; Han, J.-A.; Kweon, D.-K.; Park, J.-D.; Lim, S.-T. Effect of ultrasonic treatments on nanoparticle preparation of acid-hydrolyzed waxy maize starch. Carbohydr. Polym. 2013, 93, 582–588.
- Chen, Y.; Cao, X.; Chang, P.R.; Huneault, M.A. Comparative study on the films of poly (vinyl alcohol)/pea starch nanocrystals and poly (vinyl alcohol)/native pea starch. Carbohydr. Polym. 2008, 73, 8–17.
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and characterization of citric acid-modified starch nanoparticles/plasticized-starch composites. Biomacromolecules 2008, 9, 3314–3320.
- Tan, Y.; Xu, K.; Li, L.; Liu, C.; Song, C.; Wang, P. Fabrication of size-controlled starch-based nanospheres by nanoprecipitation. ACS Appl. Mater. Interfaces 2009, 1, 956–959.
- Le Corre, D.; Bras, J.; Dufresne, A. Starch nanoparticles: A review. Biomacromolecules 2010, 11, 1139–1153.
- Tay, S.H.; Pang, S.C.; Chin, S.F. A facile approach for controlled synthesis of hydrophilic starch-based nanoparticles from native sago starch. Starch-Stärke 2012, 64, 984–990.
- Angellier, H.; Choisnard, L.; Molina-Boisseau, S.; Ozil, P.; Dufresne, A. Optimization of the preparation of aqueous suspensions of waxy maize starch nanocrystals using a response surface methodology. Biomacromolecules 2004, 5, 1545–1551.
- Dufresne, A.; Cavaille, J.-Y.; Helbert, W. New nanocomposite materials: Microcrystalline starch reinforced thermoplastic. Macromolecules 1996, 29, 7624–7626.
- Wang, Y.; Khan, A.; Liu, Y.; Feng, J.; Dai, L.; Wang, G.; Alam, N.; Tong, L.; Ni, Y. Chitosan oligosaccharide-based dual pH responsive nano-micelles for targeted delivery of hydrophobic drugs. Carbohydr. Polym. 2019, 223, 115061.
- Liu, J.; Ai, X.; Zhang, H.; Zhuo, W.; Mi, P. Polymeric micelles with endosome escape and redox-responsive functions for enhanced intracellular drug delivery. J. Biomed. Nanotechnol. 2019, 15, 373–381.
- Liang, T.; Hou, J.; Qu, M.; Zhao, M.; Raj, I. High-viscosity α-starch nanogel particles to enhance oil recovery. RSC Adv. 2020, 10, 8275–8285.
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637.
- Chen, G.; Wei, M.; Chen, J.; Huang, J.; Dufresne, A.; Chang, P.R. Simultaneous reinforcing and toughening: New nanocomposites of waterborne polyurethane filled with low loading level of starch nanocrystals. Polymer 2008, 49, 1860–1870.
- Dufresne, A.; Castaño, J. Polysaccharide nanomaterial reinforced starch nanocomposites: A review. Starch-Stärke 2017, 69, 1500307.
- Huang, Y.; Liu, M.; Gao, C.; Yang, J.; Zhang, X.; Zhang, X.; Liu, Z. Ultra-small and innocuous cationic starch nanospheres: Preparation, characterization and drug delivery study. Int. J. Biol. Macromol. 2013, 58, 231–239.
- Zhou, G.; Luo, Z.; Fu, X. Preparation and characterization of starch nanoparticles in ionic liquid-in-oil microemulsions system. Ind. Crops Prod. 2014, 52, 105–110.
- Zhang, F.; Pei, X.; Zhai, K.; Wang, C.; Bai, Y.; Zhang, B.; Wang, Y.; Tan, Y.; Xu, K.; Wang, P. Starch-based nanospheres modified filter paper for o/w emulsions separation and contaminants removal. Int. J. Biol. Macromol. 2020, 162, 1118–1126.
- Shabana, S.; Prasansha, R.; Kalinina, I.; Potoroko, I.; Bagale, U.; Shirish, S.H. Ultrasound assisted acid hydrolyzed structure modification and loading of antioxidants on potato starch nanoparticles. Ultrason. Sonochem. 2019, 51, 444–450.
- Ji, N.; Qin, Y.; Li, M.; Xiong, L.; Qiu, L.; Bian, X.; Sun, Q. Fabrication and characterization of starch nanohydrogels via reverse emulsification and internal gelation. J. Agric. Food Chem. 2018, 66, 9326–9334.
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly (alginic acid-cl-acrylamide) nanohydrogel. Process Saf. Environ. Prot. 2017, 109, 301–310.
- Li, W.; Nie, J.; Hu, R.; Zhao, R.; Zhu, W.; Chen, X.; Li, D.; Wang, L.; Hu, L. A nanogel sensor for colorimetric fluorescence measurement of ionizing radiation doses. Chem. Commun. 2019, 55, 9614–9617.
- Noh, G.J.; Lim, S.A.; Lee, E.S. pH-responsive squeezing polysaccharidic nanogels for efficient docetaxel delivery. Polym. Adv. Technol. 2019, 30, 2067–2074.
- Lv, H.; Cui, S.; Zhang, H.; Pei, X.; Gao, Z.; Hu, J.; Zhou, Y.; Liu, Y. Crosslinked starch nanofibers with high mechanical strength and excellent water resistance for biomedical applications. Biomed. Mater. 2020, 15, 025007.
- Wang, W.; Jin, X.; Zhu, Y.; Zhu, C.; Yang, J.; Wang, H.; Lin, T. Effect of vapor-phase glutaraldehyde crosslinking on electrospun starch fibers. Carbohydr. Polym. 2016, 140, 356–361.
- Komur, B.; Bayrak, F.; Ekren, N.; Eroglu, M.; Oktar, F.N.; Sinirlioglu, Z.; Yucel, S.; Guler, O.; Gunduz, O. Starch/PCL composite nanofibers by co-axial electrospinning technique for biomedical applications. Biomed. Eng. Online 2017, 16, 40.
- Wu, D.; Samanta, A.; Srivastava, R.K.; Hakkarainen, M. Starch-Derived Nanographene Oxide Paves the Way for Electrospinnable and Bioactive Starch Scaffolds for Bone Tissue Engineering. Biomacromolecules 2017, 18, 1582–1591.
- Wang, X.; Liu, Z.; Huang, L. pH and thermo dual-responsive starch-g-P(DEAEMA-co-PEGMA): Synthesis via SET-LRP, self-assembly and drug release behaviors. React. Funct. Polym. 2019, 141, 165–171.
- Liu, W.; Li, Y.; Goff, H.D.; Nsor-Atindana, J.; Ma, J.; Zhong, F. Interfacial activity and self-assembly behavior of dissolved and granular octenyl succinate anhydride starches. Langmuir 2019, 35, 4702–4709.
- Wen, N.; Lü, S.; Xu, X.; Ning, P.; Wang, Z.; Zhang, Z.; Gao, C.; Liu, Y.; Liu, M. A polysaccharide-based micelle-hydrogel synergistic therapy system for diabetes and vascular diabetes complications treatment. Mater. Sci. Eng. C 2019, 100, 94–103.
- Ying, X.; Shan, C.; Jiang, K.; Chen, Z.; Du, Y. Intracellular pH-sensitive delivery CaCO3 nanoparticles templated by hydrophobic modified starch micelles. RSC Adv. 2014, 4, 10841–10844.
- Patnaik, S.; Panda, A.K.; Kumar, S. Thermal degradation of corn starch based biodegradable plastic plates and determination of kinetic parameters by isoconversional methods using thermogravimetric analyzer. J. Energy Inst. 2020, 93, 1449–1459.
- Da Costa, J.C.M.; Miki, K.S.L.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon 2020, 6, e03718.
- Cheng, Y.; Sun, C.; Zhai, X.; Zhang, R.; Zhang, S.; Sun, C.; Wang, W.; Hou, H. Effect of lipids with different physical state on the physicochemical properties of starch/gelatin edible films prepared by extrusion blowing. Int. J. Biol. Macromol. 2021, 185, 1005–1014.
- Qin, Y.; Yun, D.; Xu, F.; Li, C.; Chen, D.; Liu, J. Impact of storage conditions on the structure and functionality of starch/polyvinyl alcohol films containing Lycium ruthenicum anthocyanins. Food Packag. Shelf Life 2021, 29, 100693.
- Abdillah, A.A.; Charles, A.L. Characterization of a natural biodegradable edible film obtained from arrowroot starch and iota-carrageenan and application in food packaging. Int. J. Biol. Macromol. 2021, 191, 618–626.
- Busolo, M.A.; Lagaron, J.M. Oxygen scavenging polyolefin nanocomposite films containing an iron modified kaolinite of interest in active food packaging applications. Innov. Food Sci. Emerg. Technol. 2012, 16, 211–217.
- Alam, A.U.; Rathi, P.; Beshai, H.; Sarabha, G.K.; Deen, M.J. Fruit quality monitoring with smart packaging. Sensors 2021, 21, 1509.
- Terry, L.A.; Ilkenhans, T.; Poulston, S.; Rowsell, L.; Smith, A.W. Development of new palladium-promoted ethylene scavenger. Postharvest Biol. Technol. 2007, 45, 214–220.
- Smith, A.W.; Poulston, S.; Rowsell, L.; Terry, L.A.; Anderson, J.A. A new palladium-based ethylene scavenger to control ethylene-induced ripening of climacteric fruit. Platin. Met. Rev. 2009, 53, 112–122.
- Wills, R.; Warton, M. Efficacy of potassium permanganate impregnated into alumina beads to reduce atmospheric ethylene. J. Am. Soc. Hortic. Sci. 2004, 129, 433–438.
- Jedermann, R.; Praeger, U.; Geyer, M.; Lang, W. Remote quality monitoring in the banana chain. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130303.
- Mohan, C.; Ravishankar, C. Active and intelligent packaging systems—Application in seafood. World J. Aquac. Res. Dev. 2019, 1, 10–16.
- Liu, W.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Influence of processing methods and fiber length on physical properties of kenaf fiber reinforced soy based biocomposites. Compos. Part B Eng. 2007, 38, 352–359.
- Panrong, T.; Karbowiak, T.; Harnkarnsujarit, N. Thermoplastic starch and green tea blends with LLDPE films for active packaging of meat and oil-based products. Food Packag. Shelf Life 2019, 21, 100331.
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495.
- Zheng, L.; Liu, L.; Yu, J.; Shao, P. Novel trends and applications of natural pH-responsive indicator film in food packaging for improved quality monitoring. Food Control 2022, 134, 108769.
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT-Food Sci. Technol. 2015, 61, 258–262.
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324.
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control 2021, 126, 108039.
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Basha, R.K.; Naim, M.N. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602.
- Díaz-Galindo, E.P.; Nesic, A.; Bautista-Baños, S.; Dublan García, O.; Cabrera-Barjas, G. Corn-starch-based materials incorporated with cinnamon oil emulsion: Physico-chemical characterization and biological activity. Foods 2020, 9, 475.
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of active and intelligent films based on cassava starch and Chinese bayberry (Myrica rubra Sieb. et Zucc.) anthocyanins. RSC Adv. 2019, 9, 30905–30916.
- Jayakumar, A.; Heera, K.; Sumi, T.; Joseph, M.; Mathew, S.; Praveen, G.; Nair, I.C.; Radhakrishnan, E. Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food packaging application. Int. J. Biol. Macromol. 2019, 136, 395–403.
- Mustafa, P.; Niazi, M.B.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. PVA/starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J. Food Saf. 2020, 40, e12725.
- Aghazadeh, M.; Karim, R.; Sultan, M.T.; Paykary, M.; Johnson, S.K.; Shekarforoush, E. Comparison of starch films and effect of different rice starch-based coating formulations on physical properties of walnut during storage time at accelerated temperature. J. Food Process Eng. 2018, 41, e12607.
- Oliveira, B.F.; Cruz, A.; Xe, F.; Alves, E. Cassava starch coatings for postharvest control of papaya anthracnose. Phytopathol. Mediterr. 2016, 55, 276–284.
- Zhang, R.; Wang, X.; Cheng, M. Preparation and characterization of potato starch film with various size of nano-SiO2. Polymers 2018, 10, 1172.
- Castillo, L.A.; Farenzena, S.; Pintos, E.; Rodríguez, M.S.; Villar, M.A.; García, M.A.; López, O.V. Active films based on thermoplastic corn starch and chitosan oligomer for food packaging applications. Food Packag. Shelf Life 2017, 14, 128–136.
- Mali, S.; Grossmann, M.V.E. Effects of Yam Starch Films on Storability and Quality of Fresh Strawberries (Fragaria ananassa). J. Agric. Food Chem. 2003, 51, 7005–7011.
This entry is offline, you can click here to edit this entry!
