Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Post-COVID-19 respiratory manifestations comprise coughing and shortness of breath.
- post-COVID-19 syndrome
- long COVID
- long-term COVID-19
- “long hauler” syndrome
1. Introduction
While the acute symptoms of COVID-19 have been extensively reported, the longer-term effects are less well identified, because of the quite short history of the pandemic [1][2]. Specifically, most COVID-19-positive patients recover totally within 3–4 weeks after onset of infection; nevertheless, in some cases, prolonged or recurrent symptoms can be seen even weeks or months after COVID-19 recovery [3][4]. The UK’s Office for National Statistics assessed that one in five patients report symptoms beyond 5 weeks, while 10% have symptoms persevering over 12 weeks [5]. Improving the handling of these patients needs the contextualization and classification of the long-term symptoms [6]. Actually, there are varied nomenclatures and time ranges (3, 4 or 12 weeks) used to explain the condition, inadequate knowledge on its etiology and a lack of evidence for the possible treatments [6]. Indeed, various authors have used different names such as “post-COVID-19 syndrome”, “long COVID-19”, “long-term COVID-19 effects”, “long haulers” and “persistent COVID-19 symptoms” [4], which refer to various conditions such as lasting inflammation, sequelae of organ damage, hospitalization and social isolation [7]. However, the WHO has established a clinical case definition of post COVID-19 syndrome: “it occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms and that last for at least 2 months and cannot be explained by an alternative diagnosis” [8].
The original cause of the persistence of symptoms has yet to be recognized, but several hypotheses have been produced [6]: aberrant immune responses, virus-specific pathophysiological alterations, inflammatory damage in response to the acute infection [9] and mechanisms of viral persistence in certain tissues [10][11], SARS-CoV-2 interactions with host microbiome/virome communities, clotting/coagulation issues and dysfunctional brainstem/vagus nerve signaling [12]. Moreover, the roles of exosomes and mast cells [13][14][15] recently came under consideration. Furthermore, underlying risk factors can be involved: severity of early COVID-19, including symptom load, level of hospital care and necessity for mechanical ventilation [5], female gender [5], age [16][17][18][19], presence of comorbidity [18][19][20][21][22] and minority ethnicity [22][23] foster the development of long COVID.
Moreover, COVID-19 vaccines decrease the risk of contracting infection; however, studies disagree on their protective effect against long COVID [24]. Certainly, vaccines reduce the risk of long COVID by lowering the chances of contracting COVID-19 in the first place, however, for patients that do experience the infection, trials suggest that vaccination might only reduce the risk of long COVID, or have no effect on it at all [25]; consequently, long COVID can arise even after an asymptomatic coronavirus infection [24].
The respiratory system is known to be the most frequently affected by the COVID-19 acute illness phase, which is prolonged in the post-COVID-19 phase after patients’ recovery [4]. However, it is now well recognized that extrapulmonary systems such as the cardiovascular (CV) and nervous systems are also affected [4], producing symptoms such as cough, shortness of breath, fatigue, headache, brain fog, chest pains, gastrointestinal issues, joint pains and loss of taste and smell, along with neuropsychiatric symptoms, for instance, insomnia, delirium, depression and anxiety [26][27][28][29][30][31][32][33][34] (Table 1).
Table 1. Reported cardiovascular, respiratory and nervous post-COVID-19 complications.
| System Involved | Symptoms | Monitoring System |
|---|---|---|
| Cardiovascular complications |
|
|
| Respiratory complications |
|
|
| Nervous system complications |
|
|
CMR: Cardiovascular Magnetic Resonance; FEV1: Forced Expiratory Volume 1; FVC: Forced Vital Capacity; LGE: Late Gadolinium Enhancement; 6MWT: Six-Minute Walking Test; PFTs: Pulmonary Function Tests; ↑: increased; ↓: decreased.
2. Lung Involvement
The respiratory system is the primary target of COVID-19 infection, resulting in a broad spectrum of clinical and radiological manifestations. Although in about 80% of cases the infection is confined to the upper airways, in 20% the virus reaches the alveoli, leading to the formation of pulmonary infiltrates, with the onset of dyspnea, cough and fever, associated with varying degrees of hypoxemia and radiological abnormalities [35]. Interstitial pneumonia is the leading cause of hospitalization in patients with COVID-19. In most cases the disease is mild–moderate, however, progression to severe respiratory failure and acute respiratory distress syndrome (ARDS) occurs in 5–10% [36][37][38]. Among COVID-19 survivors, many patients continue to experience respiratory symptoms, and several studies have reported abnormalities in pulmonary function tests (PFTs) and chest CT images even months after hospital admission. The prevalence of these findings varies from one study to another, depending on the methodological approach and follow-up time [39][40][41]. Dyspnea and cough are the most frequently described respiratory symptoms. The biological mechanisms underlying the persistence of respiratory symptoms are not fully clear, but are probably related to the pathological processes triggered in the acute phase. Persistent endotheliopathy resulting in a pro-coagulant state and inflammatory cytokine production could be involved [40][42].
In a recent meta-analysis of 16 cohort studies with hospitalized patients, with follow-up periods > 1 month post-discharge or >2 months post-admission, the prevalence of abnormalities in lung function was approximately 20%. The most common abnormality observed was diffusion impairment, followed by restrictive ventilatory defects [39].
It is interesting to note that in several studies the presence of respiratory symptoms was not related to functional or radiological alterations. Indeed, in a subgroup of 390 patients of a large prospective cohort study, evaluated after a median of 6 months, no correlation was found between symptoms, lung function, exercise capacity and chest CT imaging. In this study, DLCO and 6-min walk distance were reduced in 29–56% and 24–29% of cases, respectively, and radiological alterations at chest CT scan were present in 41–45% of patients [26]. Moreover, in a study of 134 patients, fatigue and/or dyspnea were present in 30% of patients at 6 months of follow-up; however, these symptoms were not justified by significant abnormal findings in lung function tests or chest CT scans [43]. Furthermore, in a prospective cohort study, which enrolled 103 patients, 54% of patients had persistent dyspnea at the 3-month follow-up visit; however, most patients had lung volumes within the reference limits, while only 24% had reduced DLCO [41]. Chest CT scans showed ground-glass opacities in 25% of patients and parenchymal bands in 19% of patients [41]. However, Cortes-Telles et al. reported that patients with persistent dyspnea had reduced lung volume, lower DLCO and increased exertional desaturation, compared to those without [44]. According to the authors, persistent dyspnea could be explained by greater constraints on tidal volume expansion, exertional hypoxemia and a more rapid and shallow breathing pattern adopted by these patients [44]. The discrepancy between symptoms, lung function and imaging resulting from the studies highlights the necessity of a better understanding of the pathophysiological mechanism underlying this new pathology.
Long-term pulmonary sequelae are of particular interest in critical patients who survive COVID-19. Most published data showed a high prevalence of functional impairment and pulmonary structural abnormalities in patients requiring ICU admission [39][45][46]. Gonzalez et al. evaluated 62 patients admitted to an ICU with ARDS secondary to COVID-19 at the 3-month follow-up. Eighty-two percent of patients had reduced DLCO and 70% had signs of lung damage at CT scan. The length of invasive mechanical ventilation during the ICU stay and age were associated with the severity of radiological alterations [45]. Similar results were reported in 48 mechanically ventilated survivors of COVID-19 3 months after hospital discharge [46]. The growing attention towards these patients is also due to greater risk of developing pulmonary fibrosis than in those who had mild–moderate disease. As is known, one of the possible complications of ARDS is pulmonary fibrosis [35][47]. The risk of developing pulmonary fibrosis is related to the cellular mechanisms that occur in response to acute lung injury and can lead to abnormal and persistent inflammatory response and excessive proliferation of fibroblasts. McGroder et al. evaluated 76 patients at 4 months after hospitalization. Twenty percent of non-mechanically ventilated and 72% of mechanically ventilated patients had fibrotic-like abnormalities (reticulations, traction bronchiectasis or honeycombing) at high-resolution chest CT scan [48]. These abnormalities were correlated with decrements in lung function, cough and frailty but not with dyspnea. Furthermore, this study identified severity of initial illness, duration of mechanical ventilation, the lactate dehydrogenase levels on admission and leukocyte telomere length as independent risk factors for the development of fibrotic-like abnormalities [48]. In a prospective study reporting respiratory outcomes at 12 months after discharge in people recovered from severe COVID-19 who did not require mechanical ventilation, 24% of patients had radiological abnormalities including interstitial thickening and reticular opacity, potential signs of evolving fibrosis [49].
Specifically, steroids alone do not seem to be enough to avoid the development of fibrosis [50]. Nevertheless, it should be stated that there is not a consensus on the use of anti-fibrotics in the prevention and arresting of lung fibrosis in COVID-19 survivors yet. Nevertheless, there is a strong rationale for their potential usefulness [51]. They could be reserved for some groups of COVID-19 patients, such as the most severe ARDS cases that are most likely to end up with fibrosis [52].
3. Cardiovascular Involvement
COVID-19 affects the CV system in the acute phase, but heart complications can also arise during the post-recovery phase [4]. Specifically, reports of myocardial damage in association with COVID-19 comprise acute ischemic injury (type 1 myocardial infarction) [53], along with non-ischemic injury (i.e., myocarditis) [54][55], stress cardiomyopathy [56], heart failure (HF) [57] and secondary cardiac injury caused by sepsis and critical illness [58] (Figure 1 and Figure 2).
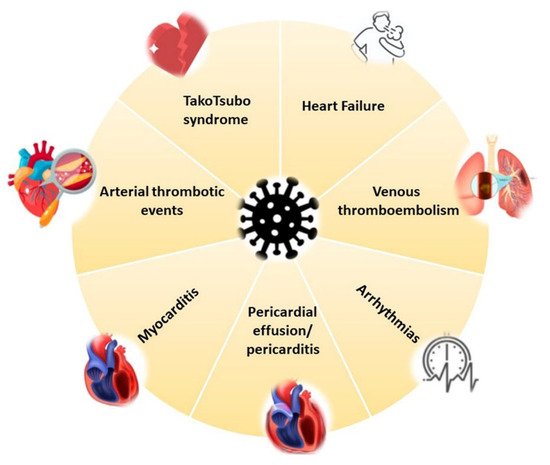
Figure 1. COVID-19 cardiovascular involvement.
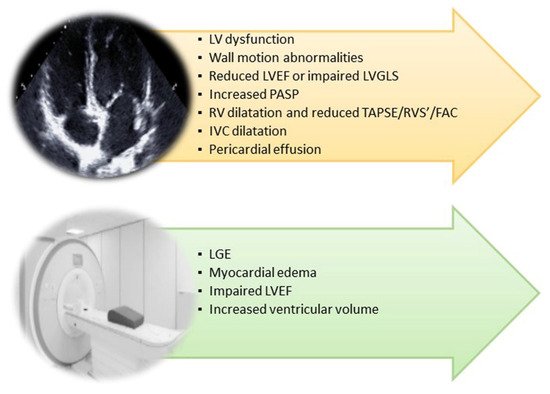
Figure 2. Cardiac imaging techniques’ main findings in post-COVID-19 syndrome. FAC: Fractional Area Change; IVC: Inferior Vena Cava; LGE: Late Gadolinium Enhancement; LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction; PASP: Pulmonary Artery Systolic Pressure; RVS’: TDI of Tricuspid Annulus; TAPSE: Tricuspid Annular Plane Systolic Excursion.
Mechanisms of myocardial injury may be indirect via systemic inflammatory response or direct (viral infection, thought to be less common) [59]. Specifically, autopsy studies on 39 COVID-19 patients identified virus in the heart tissue of 62.5% of patients [60]. The following inflammatory response may lead to cardiomyocyte death and fibro-fatty displacement of desmosomal proteins [61]. Recovered patients may have persistently increased cardiometabolic demand, as shown in long-term evaluation of SARS survivors [62], due to the reduced cardiac reserve, corticosteroid use and dysregulation of the renin–angiotensin–aldosterone system (RAAS).
Angiotensin converting enzyme 2 (ACE2) plays a crucial role in the development of CV complications [63]. Specifically, high expression of ACE2 in COVID-19 patients leads to an RAAS overactivation, with consequent dysregulation of electrolytes and fluid homeostasis [63]. Thus, excessive vasoconstriction and blood flow acceleration augment the risk of thrombosis and hypertension [64]. Moreover, high blood pressure increases the afterload on the heart and subsequently causes organic pathological changes such as cardiac dilation [65]. Myocardial fibrosis or scarring, and resultant cardiomyopathy from viral infection, can produce arrhythmias [66].
The type of acute cardiac damage that COVID-19 patients have remains uncertain. Nevertheless, there is evidence that heart attack-like events are responsible and, consequently, randomizing patients to cardioprotective medicines (NCT04333407) will help us understand the role of the CV system in COVID-19 disease. Moreover, bromodomain and extraterminal family inhibitors (BETis) improved dysfunction in human cardiac organoids (hCOs) and totally avoided cardiac dysfunction and death in a mouse cytokine storm model [67]. Furthermore, a BETi decreases transcription of genes in the viral response, reduces ACE2 expression and decreases SARS-CoV-2 infection of cardiomyocytes [67]. Together, BETis, including apabetalone, are encouraging candidates to prevent COVID-19 cardiac damage [67][68].
Palpitations and chest pain are the most common subjective findings [9]. A study by Frankfurt University Hospital revealed that 78% of survivors of COVID-19 had CV alterations, and 60% of them still showed signs of persistent myocardial inflammation more than two months after the diagnosis [69]. The results propose that long-term sequelae, for example, arrhythmias and HF, are also probable in apparently healthy people [70].
Furthermore, a study from Wuhan, China revealed that about 20% of COVID-19 patients had CV damage and the patients’ conditions would worsen if their IL-6 levels were high [71][72]. Specifically, the most severe CV complication in COVID-19 is myocarditis [73].
Myocardial damage could be the cause of an inflammatory cascade and following fibrosis; moreover, the distribution and extent of this inflammatory reaction could result in unfavorable ventricular remodeling and arrhythmias. Radin et al. showed that COVID-19 patients had prolonged relative tachycardia that lasted on average 79 days after symptom onset; specifically, 13.7% of patients did not return to resting heart rate baseline until after 133 days [74]. Furthermore, those hospitalized are at risk of even more severe sequelae, such as HF, arrhythmias, myocardial infarction and stroke (three times greater than matched controls patients) [75].
Likewise, other complications have been reported, such as postural orthostatic tachycardia syndrome [76][77] and orthostatic intolerance without hemodynamic effects [78]. Lastly, right ventricular dysfunction in response to fibrotic lung injury, pulmonary hypertension and/or clot burden in patients recovering from severe disease have also been described with an incidence of diastolic dysfunction of 32–55%, and an occurrence of pulmonary hypertension of 10–35% up to 12 weeks following the acute phase [79][80][81].
This entry is adapted from the peer-reviewed paper 10.3390/jcm11030524
References
- Scordo, K.A.; Richmond, M.M.; Munro, N. Post-COVID-19 Syndrome: Theoretical Basis, Identification, and Management. AACN Adv. Crit. Care 2021, 32, 188–194.
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631.
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; Dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Goes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700.
- Ahmad, M.S.; Shaik, R.A.; Ahmad, R.K.; Yusuf, M.; Khan, M.; Almutairi, A.B.; Alghuyaythat, W.K.Z.; Almutairi, S.B. “LONG COVID”: An insight. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5561–5577.
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427.
- Lledo, G.M.; Sellares, J.; Brotons, C.; Sans, M.; Diez Anton, J.M.; Blanco, J.; Bassat, Q.; Sarukhan, A.; Miro, J.M.; de Sanjose, S.; et al. Post-acute COVID-19 syndrome (PACS): A new tsunami requiring a universal case definition. Clin. Microbiol. Infect. 2021; in press.
- Garg, P.; Arora, U.; Kumar, A.; Wig, N. The “post-COVID” syndrome: How deep is the damage? J. Med. Virol. 2021, 93, 673–674.
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2021.
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615.
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644.
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021, 13, eabf8396.
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169.
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Mast cell activation symptoms are prevalent in Long-COVID. Int. J. Infect. Dis. 2021, 112, 217–226.
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332.
- Elrashdy, F.; Aljaddawi, A.A.; Redwan, E.M.; Uversky, V.N. On the potential role of exosomes in the COVID-19 reinfection/reactivation opportunity. J. Biomol. Struct. Dyn. 2021, 39, 5831–5842.
- Taboada, M.; Carinena, A.; Moreno, E.; Rodriguez, N.; Dominguez, M.J.; Casal, A.; Riveiro, V.; Diaz-Vieito, M.; Valdes, L.; Alvarez, J.; et al. Post-COVID-19 functional status six-months after hospitalization. J. Infect. 2021, 82, e31–e33.
- Petersen, M.S.; Kristiansen, M.F.; Hanusson, K.D.; Danielsen, M.E.; Steig, B.A.; Gaini, S.; Strom, M.; Weihe, P. Long COVID in the Faroe Islands—A longitudinal study among non-hospitalized patients. Clin. Infect. Dis. 2020, 73, e4058–e4063.
- Parente-Arias, P.; Barreira-Fernandez, P.; Quintana-Sanjuas, A.; Patino-Castineira, B. Recovery rate and factors associated with smell and taste disruption in patients with coronavirus disease 2019. Am. J. Otolaryngol. 2021, 42, 102648.
- Baricich, A.; Borg, M.B.; Cuneo, D.; Cadario, E.; Azzolina, D.; Balbo, P.E.; Bellan, M.; Zeppegno, P.; Pirisi, M.; Cisari, C.; et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: A cross-sectional study. Eur. J. Phys. Rehabil. Med. 2021, 57, 199–207.
- Stavem, K.; Ghanima, W.; Olsen, M.K.; Gilboe, H.M.; Einvik, G. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: A population-based cohort study. Thorax 2021, 76, 405–407.
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; COVID-19 BioB Outpatient Clinic Study Group; Rovere-Querini, P.; Benedetti, F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147.
- Jacobson, K.B.; Rao, M.; Bonilla, H.; Subramanian, A.; Hack, I.; Madrigal, M.; Singh, U.; Jagannathan, P.; Grant, P. Patients With Uncomplicated Coronavirus Disease 2019 (COVID-19) Have Long-Term Persistent Symptoms and Functional Impairment Similar to Patients with Severe COVID-19: A Cautionary Tale during a Global Pandemic. Clin. Infect. Dis. 2021, 73, e826–e829.
- Einvik, G.; Dammen, T.; Ghanima, W.; Heir, T.; Stavem, K. Prevalence and Risk Factors for Post-Traumatic Stress in Hospitalized and Non-Hospitalized COVID-19 Patients. Int. J. Environ. Res. Public Health 2021, 18, 2079.
- Ledford, H. Do vaccines protect against long COVID? What the data say. Nature 2021, 599, 546–548.
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55.
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232.
- Blanco, J.R.; Cobos-Ceballos, M.J.; Navarro, F.; Sanjoaquin, I.; Arnaiz de Las Revillas, F.; Bernal, E.; Buzon-Martin, L.; Viribay, M.; Romero, L.; Espejo-Perez, S.; et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin. Microbiol. Infect. 2021, 27, 892–896.
- Galvan-Tejada, C.E.; Herrera-Garcia, C.F.; Godina-Gonzalez, S.; Villagrana-Banuelos, K.E.; Amaro, J.D.L.; Herrera-Garcia, K.; Rodriguez-Quinones, C.; Zanella-Calzada, L.A.; Ramirez-Barranco, J.; Avila, J.L.R.; et al. Persistence of COVID-19 Symptoms after Recovery in Mexican Population. Int. J. Environ. Res. Public Health 2020, 17, 9367.
- Goertz, Y.M.J.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6, 542.
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6.
- Liang, L.; Yang, B.; Jiang, N.; Fu, W.; He, X.; Zhou, Y.; Ma, W.L.; Wang, X. Three-month Follow-up Study of Survivors of Coronavirus Disease 2019 after Discharge. J. Korean Med. Sci. 2020, 35, e418.
- Zhao, Y.M.; Shang, Y.M.; Song, W.B.; Li, Q.Q.; Xie, H.; Xu, Q.F.; Jia, J.L.; Li, L.M.; Mao, H.L.; Zhou, X.M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020, 25, 100463.
- Carfi, A.; Bernabei, R.; Landi, F.; The Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605.
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022.
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Li, X.; Zeng, W.; Li, X.; Chen, H.; Shi, L.; Li, X.; Xiang, H.; Cao, Y.; Chen, H.; Liu, C.; et al. CT imaging changes of corona virus disease 2019(COVID-19): A multi-center study in Southwest China. J. Transl. Med. 2020, 18, 154.
- Long, Q.; Li, J.; Hu, X.; Bai, Y.; Zheng, Y.; Gao, Z. Follow-Ups on Persistent Symptoms and Pulmonary Function Among Post-Acute COVID-19 Patients: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 702635.
- Cares-Marambio, K.; Montenegro-Jimenez, Y.; Torres-Castro, R.; Vera-Uribe, R.; Torralba, Y.; Alsina-Restoy, X.; Vasconcello-Castillo, L.; Vilaro, J. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chron. Respir. Dis. 2021, 18, 14799731211002240.
- Lerum, T.V.; Aalokken, T.M.; Bronstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.M.A.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur. Respir. J. 2021, 57, 2003448.
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553.
- Froidure, A.; Mahsouli, A.; Liistro, G.; De Greef, J.; Belkhir, L.; Gerard, L.; Bertrand, A.; Koenig, S.; Pothen, L.; Yildiz, H.; et al. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir. Med. 2021, 181, 106383.
- Cortes-Telles, A.; Lopez-Romero, S.; Figueroa-Hurtado, E.; Pou-Aguilar, Y.N.; Wong, A.W.; Milne, K.M.; Ryerson, C.J.; Guenette, J.A. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir. Physiol. Neurobiol. 2021, 288, 103644.
- Gonzalez, J.; Benitez, I.D.; Carmona, P.; Santisteve, S.; Monge, A.; Moncusi-Moix, A.; Gort-Paniello, C.; Pinilla, L.; Carratala, A.; Zuil, M.; et al. Pulmonary Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month Prospective Cohort. Chest 2021, 160, 187–198.
- van Gassel, R.J.J.; Bels, J.L.M.; Raafs, A.; van Bussel, B.C.T.; van de Poll, M.C.G.; Simons, S.O.; van der Meer, L.W.L.; Gietema, H.A.; Posthuma, R.; van Santen, S. High Prevalence of Pulmonary Sequelae at 3 Months after Hospital Discharge in Mechanically Ventilated Survivors of COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 371–374.
- Ojo, A.S.; Balogun, S.A.; Williams, O.T.; Ojo, O.S. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm. Med. 2020, 2020, 6175964.
- McGroder, C.F.; Zhang, D.; Choudhury, M.A.; Salvatore, M.M.; D’Souza, B.M.; Hoffman, E.A.; Wei, Y.; Baldwin, M.R.; Garcia, C.K. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021, 76, 1242–1245.
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754.
- Udwadia, Z.F.; Pokhariyal, P.K.; Tripathi, A.K.R.; Kohli, A. Fibrotic interstitial lung disease occurring as sequelae of COVID-19 pneumonia despite concomitant steroids. Lung India 2021, 38 (Suppl. S1), S61–S63.
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815.
- Udwadia, Z.F.; Koul, P.A.; Richeldi, L. Post-COVID lung fibrosis: The tsunami that will follow the earthquake. Lung India 2021, 38 (Suppl. S1), S41–S47.
- Bangalore, S.; Sharma, A.; Slotwiner, A.; Yatskar, L.; Harari, R.; Shah, B.; Ibrahim, H.; Friedman, G.H.; Thompson, C.; Alviar, C.L.; et al. ST-Segment Elevation in Patients with COVID-19—A Case Series. N. Engl. J. Med. 2020, 382, 2478–2480.
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824.
- Doyen, D.; Moceri, P.; Ducreux, D.; Dellamonica, J. Myocarditis in a patient with COVID-19: A cause of raised troponin and ECG changes. Lancet 2020, 395, 1516.
- van Osch, D.; Asselbergs, F.W.; Teske, A.J. Takotsubo cardiomyopathy in COVID-19: A case report. Haemodynamic and therapeutic considerations. Eur. Heart J. Case Rep. 2020, 4, 1–6.
- Chitsazan, M.; Amin, A.; Chitsazan, M.; Ziaie, N.; Amri Maleh, P.; Pouraliakbar, H.; Von Haehling, S. Heart failure with preserved ejection fraction in coronavirus disease 2019 patients: The promising role of diuretic therapy in critically ill patients. ESC Heart Fail. 2021, 8, 1610–1614.
- Jirak, P.; Larbig, R.; Shomanova, Z.; Frob, E.J.; Dankl, D.; Torgersen, C.; Frank, N.; Mahringer, M.; Butkiene, D.; Haake, H.; et al. Myocardial injury in severe COVID-19 is similar to pneumonias of other origin: Results from a multicentre study. ESC Heart Fail. 2021, 8, 37–46.
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients with COVID-19. JACC Cardiovasc. Imaging, 2021; in press.
- Lindner, D.; Fitzek, A.; Brauninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P.; et al. Association of Cardiac Infection with SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285.
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471.
- Wu, Q.; Zhou, L.; Sun, X.; Yan, Z.; Hu, C.; Wu, J.; Xu, L.; Li, X.; Liu, H.; Yin, P.; et al. Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Sci. Rep. 2017, 7, 9110.
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19.
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of COVID-19. N. Engl. J. Med. 2020, 382, 2441–2448.
- Fried, J.A.; Ramasubbu, K.; Bhatt, R.; Topkara, V.K.; Clerkin, K.J.; Horn, E.; Rabbani, L.; Brodie, D.; Jain, S.S.; Kirtane, A.J.; et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation 2020, 141, 1930–1936.
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation 2020, 142, 68–78.
- Mills, R.J.; Humphrey, S.J.; Fortuna, P.R.J.; Lor, M.; Foster, S.R.; Quaife-Ryan, G.A.; Johnston, R.L.; Dumenil, T.; Bishop, C.; Rudraraju, R.; et al. BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection. Cell 2021, 184, 2167–2182.e22.
- Robson, A. Preventing cardiac damage in patients with COVID-19. Nat. Rev. Cardiol. 2021, 18, 387.
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273.
- Kopanczyk, R.; Kumar, N.; Papadimos, T. Post-Acute COVID-19 Syndrome for Anesthesiologists: A Narrative Review and a Pragmatic Approach to Clinical Care. J. Cardiothorac. Vasc. Anesth. 2021; in press.
- Li, X.; Pan, X.; Li, Y.; An, N.; Xing, Y.; Yang, F.; Tian, L.; Sun, J.; Gao, Y.; Shang, H.; et al. Cardiac injury associated with severe disease or ICU admission and death in hospitalized patients with COVID-19: A meta-analysis and systematic review. Crit. Care 2020, 24, 468.
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810.
- Nabors, C.; Sridhar, A.; Hooda, U.; Lobo, S.A.; Levine, A.; Frishman, W.H.; Dhand, A. Characteristics and Outcomes of Patients 80 Years and Older Hospitalized With Coronavirus Disease 2019 (COVID-19). Cardiol. Rev. 2021, 29, 39–42.
- Radin, J.M.; Quer, G.; Ramos, E.; Baca-Motes, K.; Gadaleta, M.; Topol, E.J.; Steinhubl, S.R. Assessment of Prolonged Physiological and Behavioral Changes Associated With COVID-19 Infection. JAMA Netw. Open 2021, 4, e2115959.
- Wong, S.W.; Fan, B.E.; Huang, W.; Chia, Y.W. ST-segment elevation myocardial infarction in post-COVID-19 patients: A case series. Ann. Acad. Med. Singap. 2021, 50, 425–430.
- Ishibashi, Y.; Yoneyama, K.; Tsuchida, T.; Akashi, Y.J. Post-COVID-19 Postural Orthostatic Tachycardia Syndrome. Intern. Med. 2021, 60, 2345.
- Johansson, M.; Stahlberg, M.; Runold, M.; Nygren-Bonnier, M.; Nilsson, J.; Olshansky, B.; Bruchfeld, J.; Fedorowski, A. Long-Haul Post-COVID-19 Symptoms Presenting as a Variant of Postural Orthostatic Tachycardia Syndrome: The Swedish Experience. JACC Case Rep. 2021, 3, 573–580.
- Satake, K.; Hongo, M.; Ujiie, H.; Okuno, Y.; Goto, Y. The effect of cisapride on intestinal transit. Nihon Heikatsukin Gakkai Zasshi 1988, 24, 55–60.
- Bende, F.; Tudoran, C.; Sporea, I.; Fofiu, R.; Baldea, V.; Cotrau, R.; Popescu, A.; Sirli, R.; Ungureanu, B.S.; Tudoran, M. A Multidisciplinary Approach to Evaluate the Presence of Hepatic and Cardiac Abnormalities in Patients with Post-Acute COVID-19 Syndrome-A Pilot Study. J. Clin. Med. 2021, 10, 2507.
- Tudoran, C.; Tudoran, M.; Pop, G.N.; Giurgi-Oncu, C.; Cut, T.G.; Lazureanu, V.E.; Oancea, C.; Parv, F.; Ciocarlie, T.; Bende, F. Associations between the Severity of the Post-Acute COVID-19 Syndrome and Echocardiographic Abnormalities in Previously Healthy Outpatients Following Infection with SARS-CoV-2. Biology 2021, 10, 469.
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstatter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021, 57, 2003481.
This entry is offline, you can click here to edit this entry!
