The calyxes and fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino (P. alkekengi), a medicinal and edible plant, are frequently used as heat-clearing and detoxifying agents in thousands of Chinese medicine prescriptions. For thousands of years in China, they have been widely used in clinical practice to treat throat disease, hepatitis, and bacillary dysentery.
- the calyxes and fruits of P. alkekengi
- structural analysis
- quality control
- pharmacology
- pharmacokinetics
1. Introduction

2. Pharmacology
2.1. Anti-Inflammatory Activity
2.2. Anti-Tumor Activity
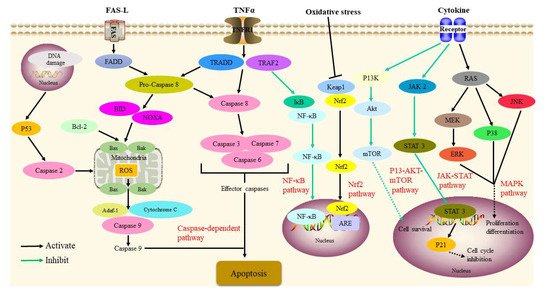
2.3. Immunosuppressive Activity
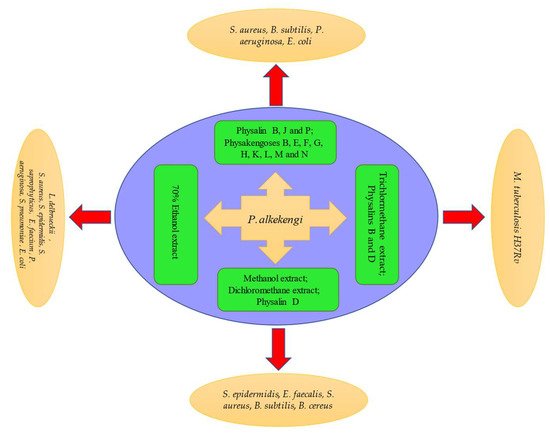
2.4. Antibacterial Activity
2.5. Antileishmanial Activity
2.6. Others
3. Summary
In summary, P. alkekengi is an excellent, abundant, inexpensive, and edible drug. The synthesis of the main active components of P. alkekengi must be further analyzed using additional biological and chemical techniques to further expand their potential applications. In addition, the quantitative analysis of the chemical constituents of P. alkekengi should be employed for the purpose of standardization and quality control of extracts. Lastly, additional in vivo animal research and clinical trials are needed to determine whether various applications of P. alkekengi are effective and safe in a larger population.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27030695
