Non-muscle-invasive bladder cancer (NMIBC) is characterized by a high rate of cure, but also by a non-negligible probability of recurrence and risk progression to muscle-invasive disease. NMIBC management requires a proper local resection and staging, followed by a risk-based treatment with intravesical agents. For many years, the current gold standard treatment for patients with intermediate or high-risk disease is transurethral resection of the bladder (TURB) followed by intravesical bacillus Calmette–Guérin (BCG) instillations. Unfortunately, in about half of high-risk patients, intravesical BCG treatment fails and NMIBC persists or recurs early. While radical cystectomy remains the gold standard for these patients, new therapeutic targets are being individuated and studied. Radical cystectomy in fact can provide an excellent long-term disease control, but can deeply interfere with quality of life. In particular, the enhanced immune checkpoints expression shown in BCG-unresponsive patients and the activity of immune checkpoints inhibitors (ICIs) in advanced bladder cancer provided the rationale for testing ICIs in NMIBC. Recently, pembrolizumab has shown promising activity in BCG-unresponsive NMIBC patients, obtaining FDA approval. Meanwhile multiple novel drugs with alternative mechanisms of action have proven to be safe and effective in NMIBC treatment and others are under investigation.
1. Immunotherapy in NMIBC: From BCG to the New Horizons of ICIs
1.1. BCG Administration Drives an Antitumour Innate and Adaptative Immune Response in NMIBC
BCG is a live attenuated strain of
Mycobacterium bovis, its activity on NMIBC was firstly demonstrated by Morales and colleagues in 1976 [
23]. The BCG mechanism of action is still not completely understood; however, it is known that BCG exposure of urothelium and bladder-resident macrophages elicits an inflammatory and immune response against tumoral cells [
24,
25,
26]. The presumed mechanism is explicated in
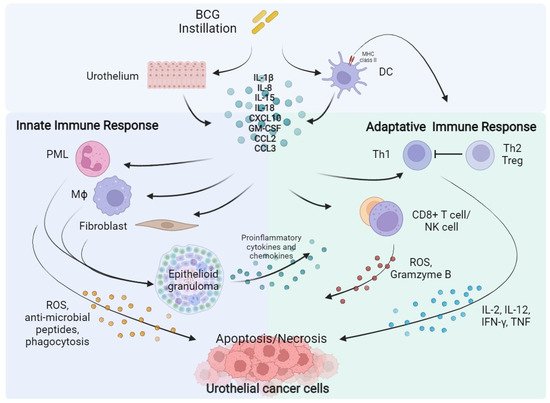
Figure 1.
Figure 1. BCG instillation elicits both innate and adaptative immune response against urothelial cancer cells. BCG, Bacillus Calmette–Guérin; DC, Dendritic Cell; IL, Interleukin; CXCL10, C-X-C motif Chemokine Ligand 10; GM-CSF, Granulocyte-Macrophage Colony-Stimulating Factor; CCL2, C-C Motif Chemokine Ligand 2; CCL3, C-C Motif Chemokine Ligand 3; PML, Polymorphonuclear Leukocytes; Th, Helper T cell; Treg, Regulatory T cell; Mϕ, Macrophage; CD8, Cluster of Differentiation 8; NK, Natural Killer; ROS, Reactive Oxygen Species; IFN-γ, Interferon-γ; TNF, Tumour Necrosis Factor.
The activation of antigen-presenting cells (APC) and urothelial cells following BCG internalization induces the release of several cytokines as Interleukin (IL)-1b, IL-8, IL-15, IL-18 and chemokine as CXC motif chemokine ligand 10 (CXCL10), Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), CC-motif Chemokine ligand 2 (CCL2), and CCL3 activating the innate and adaptive immune response [
27]. Local innate immunity activation leads to recruitment of macrophages, granulocytes, fibroblasts, dendritic cells, and lymphocytes, which form typical epithelioid and gigantocellular granulomas. In addition, neutrophils, Cluster of differentiation 8 (CD8) + T cells, and Natural Killer (NK) cells may have a direct antitumour effect, inducing the production of reactive oxygen species (ROS), antimicrobial enzymes and pro-apoptotic factors [
28,
29,
30]. Concurrently, BCG causes the expression of the major histocompatibility complex (MHC) class II presenting BCG antigens on APC and urothelial cells driving the activation of adaptative immunity [
31,
32]. A prominent T Helper (TH) 1 cells-mediated immune response, associated with the secretion of IL-2, IL-12, Interferon (IFN)-γ, and Tumour Necrosis Factor (TNF) correlates with a response to BCG instillation. On the contrary, TH2 cells activation, characterized by the releasing of IL-4, IL-5, IL-6, and IL-10, is associated with an immunosuppressive microenvironment enrich of T regulatory cells (Treg), which is associated to a BCG-unresponsive state [
24,
33].
Several randomized controlled studies and large meta-analysis have clearly demonstrated that intravesical BCG after TURB, administered with an induction schedule of 6 weekly instillations, followed by additional maintenance every 3 to 6 months over 1 to 3 years is significantly superior compared to TURB alone or TURB followed by intravesical chemotherapy in NMIBC recurrence prevention. BCG treatment provides an high rate of CR in both patients with high-risk papillary tumours and with CIS and lowers tumour progression risk, representing the standard treatment for these patients [
34].
1.2. PD-L1 and PD-1 Expression Is Associated to BCG Immune-Resistance
The main resistance mechanism to BCG treatment is linked to an intrinsic or an acquired immune resistance. The interaction of PD-1, expressed by T cells, with its ligand Programmed Death-Ligand 1 (PD-L1), normally expressed by a subset of macrophages and inducible on activated T, B and NK cells, endothelial cells, and other non-malignant cells in an inflammatory milieu is a major immune checkpoint pathway involved in immune homeostasis, down-regulating T cell response in case of chronic antigen exposure. Cancer-related overexpression of PD-L1 lets cancer cells to evade immune response, inducing T cell anergy. The use of PD-1 or PD-L1-directed mAb, can prevent their interaction and restore T cell activity against cancer cells [
35,
36].
Kates and colleagues showed in an analysis on tissue microarrays of paired pre- and post-BCG bladder samples that BCG-unresponders patients had in 25–30% of cases a pre-treatment enrichment of PD-L1 + cells, high density of CD8+ T cells, and lacked of CD4+ T cells. On the contrary, PD-L1 expression was nearly absent among BCG responders [
37]. Pierconti et al. confirmed these results. PD-L1 expression in tumour cells and in immune cells was higher in BCG-unresponsive CIS patients than in BCG-responders, suggesting that PD-L1 expression could help to identify CIS that would fail BCG therapy [
38]. In addition, BCG treatment could enhance PD-L1 and PD-1 expression. Hashizume et al. observed that PD-L1 expression levels increased after BCG. Similarly, Fukumoto et al., testing PD-1 staining in a cohort of NMIBC treated with BCG, found that PD-1 expression was superior in BCG-unresponsive tumors compared with pretreatment tumors from the same patients, hypothesizing that BCG could induce this immune checkpoint. Furthermore, PD-1 expression was correlated with worse clinical outcomes [
39,
40]. BCG instillation seems to induce the expression of PD-L1 in tumour and inflammatory cells trough the induction of CD8+ T cells, which are the major responsible of IFN-γ production [
39]. Chevalier and colleagues reported an increasing number of PD-L1-expressing CD4+ T cells (PD-L1+ Tregs) in BCG-resistant patients [
41], while Copland et al. demonstrated that BCG treatment causes the up-regulation of PD-L1 expression on APCs inducing the secretion of some cytokines as Il-6, IL-10, leading to STAT3 phosphorylation and ultimately PD-L1 expression [
42].
1.3. ICIs for the Treatment of Advanced Urothelial Cancer
The high tumoral mutational burden (TMB) of urothelial cancer, similar to melanoma and non-small-cell lung cancer, and the expression of immune checkpoint PD-1 and PD-L1 both by immune cells and microenvironmental cells constitute the biological rational for the activity of ICIs in bladder cancer [
43,
44]. Nowadays ICIs represent the standard second-line therapy in patients with advanced or metastatic urothelial cancer who progressed on first-line platinum-based chemotherapy. Pembrolizumab, an anti-PD-1 mAb, according to results of phase III trial KEYNOTE-045 is the preferred option [
45,
46]. First-line immunotherapy does not provide a statistical significant survival benefit compared to platinum-based chemotherapy, even when it was given in association; however, avelumab, an anti-PD-L1 monoclonal IgG, as maintenance in patients who did not have disease progression with first-line chemotherapy, gets the approval on the basis of JAVELIN Bladder 100 [
46,
47]. Moreover, nivolumab, another PD-1 mAb was recently granted FDA approval for the adjuvant treatment of patients with urothelial carcinoma who are at high risk of recurrence after RC on the basis of results of CheckMate-274, and several trials are investigating the role of ICIs in neoadjuvant and perioperative setting [
46,
48]. Clinical or biological markers predictive of response are still lacking; however, PD-L1 expression and elevated TMB status seem to be correlated with an increased response rate [
49].
1.4. ICIs Activity in BCG-Unresponsive NMIBC
The enhanced immune checkpoint expression shown in BCG-unresponsive patients and the efficacy of ICIs in advanced BC represented the rationale for testing them in NMIBC. Pembrolizumab was investigated in the phase II KEYNOTE-057 trial (
Table 1). In the cohort A of the study, intravenous pembrolizumab was administered for up to 24 months in patients with BCG-unresponsive CIS patients, who resulted ineligible or declines RC. After a median follow-up of 36.4 months, 41% of patients (95% CI 30.7–51.1%) achieved a CR assessed by cystoscopy and urine cytology. Eleven of 39 patients with CR (28%) were disease-free at data cut-off analysis. Results of the study cohort B, which enrolled patients with BCG-unresponsive NMIBC without CIS, have not been published yet. Safety profile was consistent with other studies testing pembrolizumab; serious treatment-related adverse events (G3 or G4 according to World Health Organization, WHO) were rare [
19]. On the basis of these results, in January 2020, FDA approved pembrolizumab for the treatment of patients with BCG-unresponsive CIS who are ineligible for or who decline RC [
19].
Atezolizumab was tested in the phase II SWOG S1605 trial (
Table 1). One hundred and thirty-five patients with BCG-unresponsive NMIBC were enrolled, 70 of them had been diagnosed with CIS, and atezolizumab was given them every 3 weeks up to complete one year of treatment. Thirty patients had a CR at 3 months (41.1%; 95% CI 29.7–53.2%) and 19 at 6 months (26.0%; 95% CI 16.5–37.6%) [
50]. In the overall population, 29 patients (29%; 90% CI 22–36%) were free of recurrence or progression at 18 months, the percentage of event-free survival was greater in non-CIS patients than in CIS patients. The treatment was globally well tolerated. Serious grade adverse events occurred in 17% of patients and there were two treatment-related deaths [
20].
Several clinical trials are now ongoing testing different ICIs in BCG-unresponsive NMIBC (Table 2). Durvalumab, an anti-PD-L1 Immunoglobulin G1 (IgG1) mAb, camrelizumab, an anti PD-1 ICI, and HX008 (pucotenlimab), a new recombinant anti-PD-1 monoclonal IgG4 are being tested as monotherapy respectively in NCT04738630, NCT04706598, NCT03759496. ADAPT-BLADDER study (NCT03317158) is investigating durvalumab activity in association with radiotherapy, while PREVERT trial (NCT03950362) activity of avelumab. Durvalumab is, furthermore, being evaluated in association with an anti-CTLA4 mAb, tremelimumab in RIDEAU study (NCT05120622). SunRISe-1 study (NCT04640623) endpoints are to assess the efficacy and safety of TAR-200, an intravesical gemcitabine-delivery system, in association with an anti PD-1 mAb, cetrelimab, or of these two drugs alone in BCG-unresponsive high-risk NMIBC. NCT04164082 trial investigates the combination of pembrolizumab and gemcitabine.
Table 2. Ongoing clinical trials testing ICIs (in bold font) alone or in combination in NMIBC.
As explicated before, BCG-resistance could be linked to an immunosuppressive state induced by the expression of immune checkpoint, and BCG itself could enhance PD-1 and PD-L1; this provides the grounds for trials that are testing anti-PD1 or anti-PD-L1 antibodies in association with BCG as front-line therapy in NMIBC in BCG-naïve patients or in patients not reaching a CR after BCG induction (
Table 2). KEYNOTE-676 (NCT03711032) is a phase III trial assessing pembrolizumab activity in combination with BCG in patients with persistent high-risk NMIBC after BCG induction. CheckMate 7G8 (NCT04149574) and POTOMAC (NCT03528694) studies testing respectively nivolumab and durvalumab have a similar design. NCT03892642 is a phase I/II trial planned to evaluate BCG in association with avelumab as induction treatment. The primary endpoint of the phase I of the trial was the completion of a full induction course. The primary endpoint was reached, the combination of BCG with an ICI was reported to be safe and well tolerated, and phase II is still ongoing [
51]. The NCT04730232 study is testing tislelizumab in association with nab-paclitaxel chemotherapy, while in the DURANCE trial (NCT04106115), it is in combination with S-488210/S-488211, a cancer multi-peptide vaccine able to stimulate a cytotoxic T cell (CTL) response against urothelial cancer cells [
52].
2. Alternative Targets: The Way to Develop New Effective Drugs
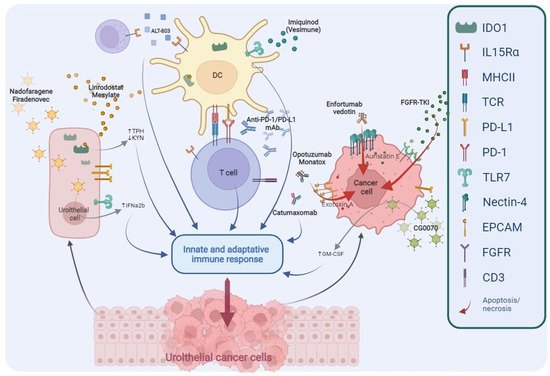
The deep improvement in the knowledge regarding the biological mechanisms responsible for neoplastic cells progression and BCG resistance mechanisms has led to identification of new targets (Figure 2); consequently, several innovative agents were developed and are now under investigation in the treatment of NMIBC (Table 3).
Figure 2. Main targets of novel drugs being investigated in BCG-unresponsive NMIBC. TPH, Tryptophan; KYN, Kynurenine; IFNα2b, Interferon α2b; DC, Dendritic Cell; PD1, Programmed cell Death protein 1; PD-L1, Programmed Death-Ligand 1; mAb, monoclonal Antibody; GM-CSF, Granulocyte-Macrophage Colony-Stimulating Factor; FGFR, Fibroblast Growth Factor Receptor; TKI, Tyrosine Kinase Inhibitors; IDO1, Indoleamine 2,3-Dioxygenase 1; IL-15Rα, Interleukin-15 receptor α; MHCII, Major Histocompatibility Complex Class II; TCR, T Cell Receptor; TLR7, Toll-like Receptor 7; EpCAM, Epithelial Cell Adhesion Molecule; CD3, Cluster of Differentiation 3.
This entry is adapted from the peer-reviewed paper 10.3390/cells11030357