Fluoride is well known for its use in the treatment of dental caries, either systemically or topically. Fluoride intake (such as in drinking water, fluoridated toothpaste, or fluoride supplements) is the cornerstone to preventing dental caries in adults and children. Fluoride prevents dental caries by slowing down the demineralization of enamel, which is caused by the interaction between dental plaque and dental hard tissues. Fluoride may inhibit tooth decay by 40–60% by co-precipitating calcium and phosphate ions and by enhancing the precipitation of fluoridated apatite. Fluoride is also found deposited as calcium fluoride in dental plaque, which helps to prevent dental caries.
- fluoride
- dentistry
- osteoblasts
- osteoclasts
1. Fluoride and Epithelial Cells
1.1. High Concentration Fluoride and Epithelial Cells
1.2. Low Concentration Fluoride and Epithelial Cells
1.3. Fluoride and Epithelial-Mesenchymal Interactions
2. Fluoride and Bone Marrow Mesenchymal Stem Cells (BMMSCs)
3. Fluoride and Bone Metabolism
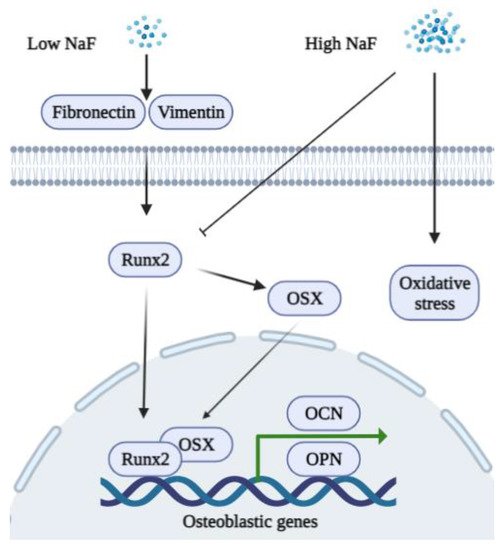
3.1. Fluoride and Osteoblasts
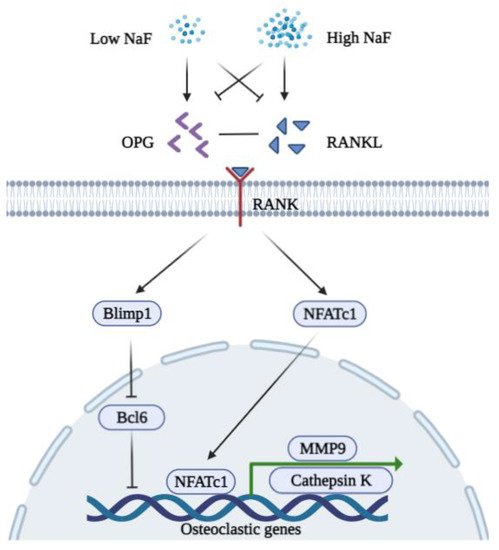
3.2. Fluoride and Osteoclasts
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020962
References
- Kawano, S.; Saito, M.; Handa, K.; Morotomi, T.; Toyono, T.; Seta, Y.; Nakamura, N.; Uchida, T.; Toyoshima, K.; Ohishi, M.; et al. Characterization of Dental Epithelial Progenitor Cells Derived from Cervical-loop Epithelium in a Rat Lower Incisor. J. Dent. Res. 2004, 83, 129–133.
- Crivelini, M.M.; Oliveira, D.T.; De Mesquita, R.A.; De Sousa, S.C.O.M.; Loyola, A.M. Kallikrein 4 and matrix metalloproteinase-20 immunoexpression in malignant, benign and infiltrative odontogenic tumors. J. Oral Maxillofac. Pathol. 2016, 20, 246–251.
- Mu, Y.; Tian, R.; Xiao, L.; Sun, D.; Zhang, Z.; Xu, S.; Yang, G. Molecular Evolution of Tooth-Related Genes Provides New Insights into Dietary Adaptations of Mammals. J. Mol. Evol. 2021, 89, 458–471.
- Wei, W.; Gao, Y.; Wang, C.; Zhao, L.; Sun, D. Excessive fluoride induces endoplasmic reticulum stress and interferes enamel proteinases secretion. Environ. Toxicol. 2011, 28, 332–341.
- DenBesten, P.K.; Zhu, L.; Li, W.; Tanimoto, K.; Liu, H.; Witkowska, H.E. Fluoride incorporation into apatite crystals delays amelogenin hydrolysis. Eur. J. Oral Sci. 2011, 119, 3–7.
- DenBesten, P.K.; Yan, Y.; Featherstone, J.D.B.; Hilton, J.F.; Smith, C.E.; Li, W. Effects of fluoride on rat dental enamel matrix proteinases. Arch. Oral Biol. 2002, 47, 763–770.
- Sharma, R.; Tsuchiya, M.; Skobe, Z.; Tannous, B.A.; Bartlett, J.D. The Acid Test of Fluoride: How pH Modulates Toxicity. PLoS ONE 2010, 5, e10895.
- Gao, J.; Ruan, J.; Gao, L. Excessive fluoride reducesFoxo1expression in dental epithelial cells of the rat incisor. Eur. J. Oral Sci. 2014, 122, 317–323.
- Zuckerbraun, H.L.; Babich, H.; May, R.; Sinensky, M.C. Triclosan, cytotoxicity, mode of action, and induction of apoptosis in human gingival cells in vitro. Eur. J. Oral Sci. 1998, 106, 628–636.
- Yan, Q.; Zhang, Y.; Li, W.; DenBesten, P. Micromolar Fluoride Alters Ameloblast Lineage Cells in vitro. J. Dent. Res. 2007, 86, 336–340.
- Jorgensen, S.N.; Sanders, J.R. Mathematical models of wound healing and closure: A comprehensive review. Med. Biol. Eng. Comput. 2015, 54, 1297–1316.
- Arakawa, Y.; Bhawal, U.K.; Ikoma, T.; Kimoto, K.; Kuroha, K.; Kubota, T.; Hamada, N.; Kubota, E.; Arakawa, H. Low concentration fluoride stimulates cell motility of epithelial cells in vitro. Biomed. Res. 2009, 30, 271–277.
- Wixler, V. The role of FHL2 in wound healing and inflammation. FASEB J. 2019, 33, 7799–7809.
- Cho, Y.-D.; Kim, K.-H.; Lee, Y.-M.; Ku, Y.; Seol, Y.-J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456.
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.-S.; Li, X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 79.
- El-Baz, L.M.; Shoukry, N.M.; Hafez, H.S.; Guzy, R.D.; Salem, M.L. Fibroblast Growth Factor 2 Augments Transforming Growth Factor Beta 1 Induced Epithelial-mesenchymal Transition in Lung Cell Culture Model. Iran. J. Allergy Asthma Immunol. 2020, 19, 348–361.
- Kinoshita-Ise, M.; Tsukashima, A.; Kinoshita, T.; Yamazaki, Y.; Ohyama, M. Altered FGF expression profile in human scalp-derived fibroblasts upon WNT activation: Implication of their role to provide folliculogenetic microenvironment. Inflamm. Regen. 2020, 40, 1–10.
- Meng, J.; Chen, S.; Han, J.-X.; Qian, B.; Wang, X.-R.; Zhong, W.-L.; Qin, Y.; Zhang, H.; Gao, W.-F.; Lei, Y.-Y.; et al. Twist1 Regulates Vimentin through Cul2 Circular RNA to Promote EMT in Hepatocellular Carcinoma. Cancer Res. 2018, 78, 4150–4162.
- He, D.; Bhawal, U.K.; Hamada, N.; Kuboyama, N.; Abiko, Y.; Arakawa, H. Low Level Fluoride Stimulates Epithelial-Mesenchymal Interaction in Oral Mucosa. J. Hard Tissue Biol. 2013, 22, 59–66.
- Chen, F.; Han, Y.; Kang, Y. Bone marrow niches in the regulation of bone metastasis. Br. J. Cancer 2021, 214, 1912–1920.
- Zhang, R.; Li, X.; Liu, Y.; Gao, X.; Zhu, T.; Lu, L. Acceleration of Bone Regeneration in Critical-Size Defect Using BMP-9-Loaded nHA/ColI/MWCNTs Scaffolds Seeded with Bone Marrow Mesenchymal Stem Cells. BioMed Res. Int. 2019, 2019, 7343957.
- Bhawal, U.K.; Li, X.; Suzuki, M.; Taguchi, C.; Oka, S.; Arikawa, K.; Tewari, N.; Liu, Y. Treatment with low-level sodium fluoride on wound healing and the osteogenic differentiation of bone marrow mesenchymal stem cells. Dent. Traumatol. 2019, 36, 278–284.
- Garcia, A.L.; Picinini, J.; Silveira, M.D.; Camassola, M.; Visentim, A.P.; Salvador, M.; da Silva, J. Fluorosilicic acid induces DNA damage and oxidative stress in bone marrow mesenchymal stem cells. Mutat. Res. Toxicol. Environ. Mutagen. 2020, 861–862, 503297.
- Fu, X.; Xie, F.-N.; Dong, P.; Li, Q.-C.; Yu, G.-Y.; Xiao, R. High-Dose Fluoride Impairs the Properties of Human Embryonic Stem Cells via JNK Signaling. PLoS ONE 2016, 11, e0148819.
- Xu, S.; Xie, X.; Li, C.; Liu, Z.; Zuo, D. Micromolar sodium fluoride promotes osteo/odontogenic differentiation in dental pulp stem cells by inhibiting PI3K/AKT pathway. Arch. Oral Biol. 2021, 131, 105265.
- Pan, Y.; Li, Z.; Wang, Y.; Yan, M.; Wu, J.; Beharee, R.G.; Yu, J. Sodium fluoride regulates the osteo/odontogenic differentiation of stem cells from apical papilla by modulating autophagy. J. Cell. Physiol. 2019, 234, 16114–16124.
- Ngoc, T.D.N.; Son, Y.-O.; Lim, S.-S.; Shi, X.; Kim, J.-G.; Heo, J.S.; Choe, Y.; Jeon, Y.-M.; Lee, J.-C. Sodium fluoride induces apoptosis in mouse embryonic stem cells through ROS-dependent and caspase- and JNK-mediated pathways. Toxicol. Appl. Pharmacol. 2012, 259, 329–337.
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245.
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast–osteoclast interactions. Connect. Tissue Res. 2017, 59, 99–107.
- Sims, N.A.; Martin, T.J. Osteoclasts Provide Coupling Signals to Osteoblast Lineage Cells Through Multiple Mechanisms. Annu. Rev. Physiol. 2020, 82, 507–529.
- Antonarakis, G.S.; Moseley, R.; Waddington, R.J. Differential influence of fluoride concentration on the synthesis of bone matrix glycoproteins within mineralizing bone cellsin vitro. Acta Odontol. Scand. 2014, 72, 1066–1069.
- Simon, M.J.K.; Beil, F.T.; Riedel, C.; Lau, G.; Tomsia, A.; Zimmermann, E.A.; Koehne, T.; Ueblacker, P.; Rüther, W.; Pogoda, P.; et al. Deterioration of teeth and alveolar bone loss due to chronic environmental high-level fluoride and low calcium exposure. Clin. Oral Investig. 2016, 20, 2361–2370.
- Liu, S.; Zhou, H.; Liu, H.; Ji, H.; Fei, W.; Luo, E. Fluorine-contained hydroxyapatite suppresses bone resorption through inhibiting osteoclasts differentiation and function in vitro and in vivo. Cell Prolif. 2019, 52, e12613.
- Yamaguchi, M. Fluoride and bone metabolism. Clin. Calcium 2007, 17, 217–223.
- Kurdi, M.S. Chronic fluorosis: The disease and its anaesthetic implications. Indian J. Anaesth. 2016, 60, 157–162.
- Qiao, W.; Liu, Q.; Li, Z.; Zhang, H.; Chen, Z. Changes in physicochemical and biological properties of porcine bone derived hydroxyapatite induced by the incorporation of fluoride. Sci. Technol. Adv. Mater. 2017, 18, 110–121.
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The Effect of Inflammation on Bone. Front. Physiol. 2021, 11.
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2015, 82, 42–49.
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24.
- Tang, C.-Y.; Wu, M.; Zhao, D.; Edwards, D.; McVicar, A.; Luo, Y.; Zhu, G.; Wang, Y.; Zhou, H.-D.; Chen, W.; et al. Runx1 is a central regulator of osteogenesis for bone homeostasis by orchestrating BMP and WNT signaling pathways. PLoS Genet. 2021, 17, e1009233.
- Takahata, Y.; Hagino, H.; Kimura, A.; Urushizaki, M.; Kobayashi, S.; Wakamori, K.; Fujiwara, C.; Nakamura, E.; Yu, K.; Kiyonari, H.; et al. Smoc1 and Smoc2 regulate bone formation as downstream molecules of Runx2. Commun. Biol. 2021, 4, 1–11.
- Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Bononi, I.; Frontini, F.; Mazzoni, E.; Oton-Gonzalez, L.; Rotondo, J.C.; Torreggiani, E.; Tognon, M.; et al. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 2021, 11, 6573–6591.
- Duan, X.-Q.; Zhao, Z.-T.; Zhang, X.-Y.; Wang, Y.; Wang, H.; Liu, D.-W.; Li, G.-S.; Jing, L. Fluoride Affects Calcium Homeostasis and Osteogenic Transcription Factor Expressions Through L-type Calcium Channels in Osteoblast Cell Line. Biol. Trace Element Res. 2014, 162, 219–226.
- Lee, M.; Arikawa, K.; Nagahama, F. Micromolar Levels of Sodium Fluoride Promote Osteoblast Differentiation Through Runx2 Signaling. Biol. Trace Element Res. 2017, 178, 283–291.
- Volobaev, V.P.; Serdyukova, E.S.; Kalyuzhnaya, E.E.; Schetnikova, E.A.; Korotkova, A.D.; Naik, A.A.; Bach, S.N.; Prosekov, A.Y.; Larionov, A.V. Investigation of the genotoxic effects of fluoride on a bone tissue model. Toxicol. Res. 2020, 36, 337–342.
- Burgener, D.; Bonjour, J.-P.; Caverzasio, J. Fluoride increases tyrosine kinase activity in osteoblast-like cells: Regulatory role for the stimulation of cell proliferation and Pi transport across the plasma membrane. J. Bone Miner. Res. 2009, 10, 164–171.
- Farley, J.R.; Wergedal, J.E.; Baylink, D.J. Fluoride Directly Stimulates Proliferation and Alkaline Phosphatase Activity of Bone-Forming Cells. Science 1983, 222, 330–332.
- Pak, C.Y.; Zerwekh, J.E.; Antich, P. Anabolic effects of fluoride on bone. Trends Endocrinol. Metab. 1995, 6, 229–234.
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The Role of Osteoprotegerin and Its Ligands in Vascular Function. Int. J. Mol. Sci. 2019, 20, 705.
- Yang, S.; Wang, Z.; Farquharson, C.; Alkasir, R.; Zahra, M.; Ren, G.; Han, B. Sodium fluoride induces apoptosis and alters bcl-2 family protein expression in MC3T3-E1 osteoblastic cells. Biochem. Biophys. Res. Commun. 2011, 410, 910–915.
- Zhao, W.-P.; Wang, H.-W.; Liu, J.; Zhang, Z.-H.; Zhu, S.-Q.; Zhou, B.-H. Mitochondrial respiratory chain complex abnormal expressions and fusion disorder are involved in fluoride-induced mitochondrial dysfunction in ovarian granulosa cells. Chemosphere 2018, 215, 619–625.
- Wu, S.; Yan, W.; Qiu, B.; Liao, Y.; Gu, J.; Wei, S.; Zhang, A.; Pan, X. Aberrant methylation-induced dysfunction of p16 is associated with osteoblast activation caused by fluoride. Environ. Toxicol. 2018, 34, 37–47.
- Simonet, W.; Lacey, D.; Dunstan, C.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319.
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208.
- McDonald, M.M.; Kim, A.S.; Mulholland, B.S.; Rauner, M. New Insights Into Osteoclast Biology. JBMR Plus 2021, 5, e10539.
- Zhao, X.; Patil, S.; Xu, F.; Lin, X.; Qian, A. Role of Biomolecules in Osteoclasts and Their Therapeutic Potential for Osteoporosis. Biomolecules 2021, 11, 747.
- Kitamura, N.; Kaminuma, O. Isoform-Selective NFAT Inhibitor: Potential Usefulness and Development. Int. J. Mol. Sci. 2021, 22, 2725.
- Bhawal, U.K.; Lee, H.-J.; Arikawa, K.; Shimosaka, M.; Suzuki, M.; Toyama, T.; Sato, T.; Kawamata, R.; Taguchi, C.; Hamada, N.; et al. Micromolar sodium fluoride mediates anti-osteoclastogenesis in Porphyromonas gingivalis-induced alveolar bone loss. Int. J. Oral Sci. 2015, 7, 242–249.
- Okuda, A.; Kanehisa, J.; Heersche, J.N.M. The effects of sodium fluoride on the resorptive activity of isolated osteoclasts. J. Bone Miner. Res. 2010, 5, S115–S120.
- Łukomska, A.; Baranowska-Bosiacka, I.; Dec, K.; Pilutin, A.; Tarnowski, M.; Jakubczyk, K.; Żwierełło, W.; Skórka-Majewicz, M.; Chlubek, D.; Gutowska, I. Changes in Gene and Protein Expression of Metalloproteinase-2 and -9 and their Inhibitors TIMP2 and TIMP3 in Different Parts of Fluoride-Exposed Rat Brain. Int. J. Mol. Sci. 2020, 22, 391.
- Cvikl, B.; Lussi, A.; Carvalho, T.S.; Moritz, A.; Gruber, R. Stannous chloride and stannous fluoride are inhibitors of matrix metalloproteinases. J. Dent. 2018, 78, 51–58.
- Miyauchi, Y.; Ninomiya, K.; Miyamoto, H.; Sakamoto, A.; Iwasaki, R.; Hoshi, H.; Miyamoto, K.; Hao, W.; Yoshida, S.; Morioka, H.; et al. The Blimp1–Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J. Exp. Med. 2010, 207, 751–762.
- Yao, Y.; Ma, Y.; Zhong, N.; Pei, J. The Inverted U-Curve Association of Fluoride and Osteoclast Formation in Mice. Biol. Trace Elem. Res. 2019, 191, 419–425.
- Jiang, N.; Guo, F.; Xu, W.; Zhang, Z.; Jin, H.; Shi, L.; Zhang, X.; Gao, J.; Xu, H. Effect of fluoride on osteocyte-driven osteoclastic differentiation. Toxicology 2020, 436, 152429.
- Jiang, N.; Guo, F.; Sun, B.; Zhang, X.; Xu, H. Different Effects of Fluoride Exposure on the Three Major Bone Cell Types. Biol. Trace Elem. Res. 2019, 193, 226–233.
