Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
|
Area Studies
Nuclear power is generated by a small amount of fuel, as related to other non-renewable energy sources. The volume of waste formed in this process is comparably small. Thermal treatment of waste is a term given to any treatment technology that involves high temperatures in the processing of the waste feedstock. All the thermal treatment methods used for normal waste have been applied to radioactive waste.
- radioactive waste
- thermal treatment
- safety of the radioactive waste
- decontamination
- mass reduction
1. Introduction
Radioactive waste is defined as solid, liquid, or gaseous material containing or contaminated with radioactive substances in concentrations or activities exceeding the limit values settled by national regulating bodies, the use of which is not anticipated or considered [1]. Besides by nuclear power plants, it is produced by the scientific laboratories, nuclear medicine centers, and the non-nuclear industry that apply the radioactive sources. As the radioactive waste emits the ionizing radiation, it causes a particular risk to human health and to the environment [2].
Basing on the radioactivity concentration of radioactive isotopes present in the waste, it is classified as:
- ✓
-
low active,
- ✓
-
medium active, or
- ✓
-
highly active.
All the above categories of radioactive waste can be divided into subcategories, according to the half-life of radioactive isotopes present in this waste.
The criteria for dividing radioactive waste into categories and subcategories are presented in Table 1 [3][4].
| Categories * | Subcategories | |||
|---|---|---|---|---|
| Temporary (Transition) | Short-Lived | Long-Lived | ||
| Low-level waste | LAC < AC ≤ 104 LAC |
|
t1/2 ≤ 30 years
t1/2 30 years
|
t1/2 > 30 years AC > 400 kBq/kg for long-lived isotopes |
| Intermediate waste | 104 LAC < AC ≤ 107 LAC | |||
| High-level waste | AC > 107 LAC | |||
*/AC (activity concentration)—radioactive concentration of the isotope in the waste (kBq/kg), LAC (legal activity concentration)—the value of the radioactive isotope concentration, which is the basis for classifying the waste as radioactive waste (kBq/kg).
The spent nuclear fuel and products of nuclear fuel production and processing plants intended for disposal are classified as highly active radioactive waste.
Radioactive waste of low and intermediate level (LLW and ILW, respectively) from nuclear power plants is a wide range of items and materials, ranging from rubber gloves and protective covers for footwear (LLW), through wastewater from cleaning procedures of water from cooling circuits of the power plant and other solid intermediate level waste (ILW). They are akin to industrial and medical waste that people have many years of experience with. Therefore, most of the industrial forms of treatment and storage of municipal waste can be applied to radioactive waste. Unlike other hazardous industrial waste materials, hazard menace of the radioactive waste that results from its radioactivity decreases over time. Therefore, the management of low- and intermediate-level radioactive waste, especially if they have a small mass, often consists only of the effective conditioning, shielding and storage for several decades (in the UK, one hundred years) in specially constructed bunkers.
2. Thermal Methods of the Radioactive Waste Immobilization
If radioactive waste is stored in liquid form, it may enter the environment by leaching with numerous aqueous streams. In a solid form, risk of the radioactive matter by leaching is many times smaller. Waste solidifying may be reached primarily by its chemical incorporating into the structure of an appropriate matrix, e.g., of cement or glass, and is mainly used for high-level waste (HLW). Physical stabilization of waste is mainly realized by using materials that do not form chemical bonds with the radionuclides, e.g., bitumen or cement. It is used for intermediate level radioactive waste (ILW), as well as for HLW.
Selection the proper form (matrix) for immobilizing radioactive waste is a problem far-reaching beyond its durability. Priority is given to use reliable, simple, cheap, and easy operating methods; only in certain cases should costly or complex ones be used. The choice of immobilization technology should also consider the physical and chemical nature of the waste, as well as type of storage and disposal facility to which the waste will be sent [5].
Below, we present three immobilization methods that are realized in the elevated temperatures.
2.1. Incorporation into Bitumen (Bituminization)
The technological process of bituminization the radioactive waste permits forming solid blocks containing both liquid or solid radioactive waste and being a good choice for safe, long-term storage and disposal [6]. The process is preferably used for medium-activate waste, mainly chemical sludges, concentrates from evaporators, solutions arising from an ion-exchange purified liquid waste, organic solvents, and used natural and synthetic sorbents, ash, and plastics. The first industrial-scale plant started operation in 1965 in Belgium. Since then, bituminization has been introduced in many countries, such as the UK, Czechoslovakia, France, Germany, Poland, Russian Federation, and the USA, among others.
Bitumen is a thermoplastic organic, solid, or semi-solid material in low and medium temperatures and is formed by mixtures of different high molecular weight hydrocarbons that are soluble in carbon disulphide. They contain also small quantities of sulfur, nitrogen, and oxygen compounds and traces of other elements, such as metals. The lowest temperature at which volatile bitumen components begin to burn with an open flame under normal conditions (flash point) cannot be reached during waste treatment for safety reasons. Bitumen used as matrix material has the relatively high flashpoint, usually in the range of 250 to 300 °C.
High stability of bitumen with respect to alpha, beta, or gamma radiation is an important feature determining:
- ✓
-
the maximum loading amount of radioactive waste into the bitumen filled containers and
- ✓
-
behavior of the final form of waste under storage and disposal conditions.
There are two basic types of the bituminization process: batch or continuous method. The first one is preferably used for processing portioned waste to prepare a certain number of packages of conditioned waste. Continuous bitumen processing, in turn, is used to form solid waste streams in the process facilities working repeatedly. In both cases, the resulting conditioned solid product is ready for long lasting, safe storage and, further, for disposal.
Batch processes, in general, consist of two stages: (1) drying either liquid or solid radioactive waste portions and (2) mixing the dried material with heated (usually to 200–230 °C), molten bitumen. The mass of these mixed portions of solid residue depends on the type and mass of the bitumen. Walls of the mixing vessel, in many cases, are additionally heated to about 300 °C. In such conditions, most of the water evaporates, and particles of the solid residue are suspended in the bitumen. The resulting mixture is stirred further in the elevated temperature to evaporate the excess of water and then poured into metal barrels (hobocks) and cooled for solidifying contents of the drums. A simplified diagram of the batch bituminization installation operating in the reprocessing plant at the Research Center for the Applications of Nuclear Energy (SCK CEN, Mol, Belgium) is shown in Figure 1.
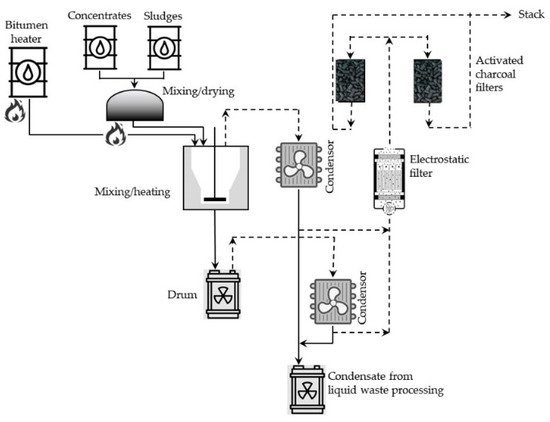
Figure 1. Simplified diagram of the batch bituminization installation operating in the reprocessing plant at SCK CEN, Mol, Belgium (adapted from Ref. [7]).
At present, batch process is not commonly used because bituminization installations operating in the continuous mode seem to be less complicated and cheaper. In this method, the waste stream is continuously delivered at a constant rate to hot bitumen and mixed to form a uniform product. Then, final matter is poured into steel containers in a constant flow rate. Two versions of the continuous processes are the extrusion method and thin film evaporation.
In the case of extrusion systems, concentrates are introduced continuously into a screw extruder, simultaneously with bitumen, heated at 130–200 °C. When passing through the extruder, the waste is thoroughly mixed with bitumen, and water is simultaneously evaporated. The somewhat low temperature (130–140 °C) of the bitumen-waste mixture has been found to be high enough to keep the viscosity at the appropriate value for the extrusion process. The final product is a homogenous suspension of mineral particles of waste coated with a layer of bitumen mixed with a bitumenic solution of the soluble components of waste. Then, the final mixture is poured into hobocks and left to cool and solidify. A simplified diagram of (continuous) extruder-type bituminization is presented in Figure 2.
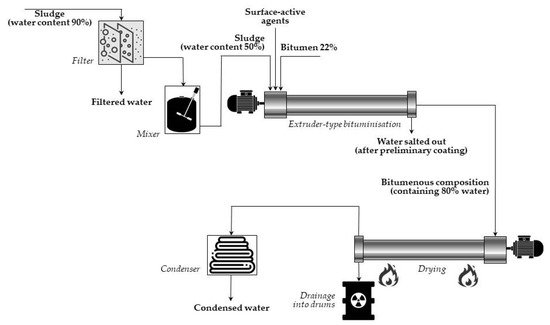
Figure 2. Simplified diagram of (continuous) extruder-type bituminization made in Marcoule, France (adapted from Ref. [8]).
Since 1965, when the first extruding installation started operation at the Commissariat a l’Energie Atomique (CEA, Marcoule, France), a large number such facilities have been created world-wide for processing different concentrates from liquid radioactive waste.
In the thin film evaporating process, liquid waste and/or organic ion exchangers are first concentrated to obtain material containing about 60% of dry matter. Next, preheated waste sludge and bitumen are put separately at the top of the thin-film rotary evaporator, mixed, and both streams move along the heated compartment. There, residual water present in the mixture is gradually evaporated, and the mineral residues from the waste are mixed with bitumen. Finally, the bituminous mix of waste is poured at the bottom into the steel containers, and the generated vapor is condensed in a set of condensers. One of the first thin-film evaporators (Luwa L 150) for bitumen coating of radioactive concentrates was constructed in the CEA Production of Plutonium Center in Marcoule (France) in 1969 [7].
The only bituminization installation in Poland operates in the Radioactive Waste Management Plant (RWMP) located in Otwock-Swierk. The waste pre-treatment is performed in the evaporation facility, in the reverse osmosis installation, or in a tank for sorption purifying aqueous wastes using synthetic inorganic sorbents. After such pre-treatment, solid concentrates are bituminized: radioactive sludge is mixed with bitumen at a temperature of 220–250 °C. As a result, about 99% of the water contained in the sludge is evaporated before the product is poured into the metal storage drums. An important problem in the ongoing process is the need to keep a high temperature of the mixture (up to 250 °C) inside the tank and even higher temperature (up to 300 °C) at its walls. Such conditions may result in thermal decomposition of the bitumen and the waste, spontaneous combustion, or explosion of the content of the drum [9]. The installation for the bituminization operates with the non-emulsified bitumen of the P-60 or D-35 type [10].
Ultimately, the profits of using bitumen are [11]:
- ✓
-
the device is simple,
- ✓
-
the used material is relatively inexpensive,
- ✓
-
temperature of the process is relatively low,
- ✓
-
bitumen can form a homogeneous mixture with waste of different composition, and
- ✓
-
volume of the final product is even 5 times smaller than in the cementation.
The main disadvantage is that the bitumen is easily combustible, and there is a possibility that it may come into an exothermic interaction with some components of waste at high temperature. Decreasing content in the waste of the compounds that may oxidize bitumen at processing temperature (e.g., metal nitrates) may protect the bitumen mixture from the dangerous ignition of stored waste.
2.2. Vitrification the Radioactive Waste
Vitrification (in the UK, or glassification, in the United States) is the conversion of a material into a glassy form to stabilize radioactive waste before storing it. Currently, the process is the most important waste solidification process. Vitrification has been studied since the 1950s, when the nuclear industry was started. Initially, natural soils were used as a material for forming glass [12]. However, the necessity of heating to high temperatures (~1500 °C), required for glass melting and producing homogeneous, as well as bubble-free end-product, has resulted in searching for alternative glasses. At present, borosilicate glass of lower melting point, and which accept up to 40% by weight of waste, was proposed as the best solution. In the Soviet Union, however, phosphate glass was applied for vitrification waste. Later, in Russia, use of borosilicate glass vitrification technology has been also applied. The search for suitable glasses is still ongoing. The studies are also conducted in Poland: at the AGH University of Science and Technology (Cracow) [13] and at the Institute of Chemistry and Nuclear Technology (Warsaw) [14].
At present, vitrification is carried out by heating to high temperature and rapid cooling in both in-situ and ex-situ modes (for the contaminated soil or for high level liquid waste (HLLW) from the nuclear fuel reprocessing, respectively).
Removal of radioactively contaminated soils could induce radiation exposure to workers from dust and fugitive gas emissions, resulting in an increase in the risk to the environment. A good solution to prevent this seems to be the in-situ vitrification (ISV), which uses an electric power for heating the soil to an extremely high temperature (in the range of 1600–2000 °C) necessary for heating and melting it. For this purpose, two electrodes are placed in a contaminated ground, and an electric current is delivered. Melting begins in the surface zone of the earth and moves downwards. As the soil melts, the electrodes descend into the solid part, causing melting of the sequent layers of soil. When the power is turned off, the molten soil cools down and turns into a glass-like block with the electrodes remaining in it. Any radioactive matter is trapped in this glass bloc and may stand underground for a long time, leaching protected. The method is highly effective for near-surface contamination, and modern methods of vitrification allow for treatment up to depths of about 10 m.
Ex-situ vitrification (ESV) resembles ISV; however, it is carried out in specially designed chambers outside the place of contamination or the storing place of waste. Heating elements of the melter are either plasma torches or electric arc furnaces. When the plasma torch technology is used, waste and the silica flux are fed into a rotating hearth and placed on the chambers’ walls by centrifugal force. On rotation, the waste passes through the plasma flux generated by the immobile plasma burner. To remove molten material from the furnace, the rotation is slowed down, and the slag is poured through the valve in the bottom. Effluent gases, in turn, enter a separate vessel where they are oxidated by combustion. The arc furnace, in turn, is equipped with carbon electrodes and cooled sidewalls, able to work by a continuous feeding system and a system for treatment of an off gas. In this version of the process, the waste is loaded into a chamber and heated to a temperature sufficient to melt the glass. After excretion, the vitrificated waste is cooled down to form a storage-suitable product.
In its full pattern, vitrification of radioactive waste is a multi-stage process: first, the waste is mixed with a substance that crystallizes upon heating (e.g., sugar, sand), and the mixture is calcined to remove water and increase stability of the glassy product. Then, the calcined materials enter the heated furnace as a continuous stream and are mixed with crushed glass to bond the radioactive waste with glass. Finally, the molten product is poured into an encapsulation container, cooled down, and solidified in a final glass suitable for storage. Such a vitrification process allows the dangerous radioactive waste to be immobilized for thousands of years. A simplified flowsheet of the vitrification plant at Marcoule (AVM) is presented in Figure 3 [15][16]. In the process, temperature of heating the melter is about 1050 °C.
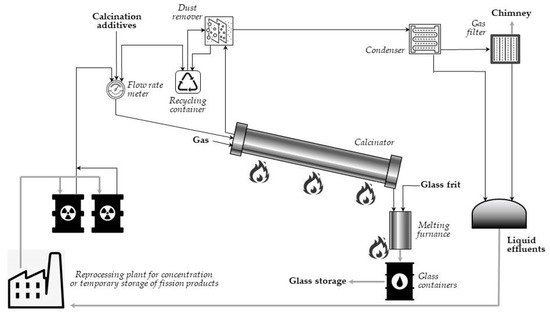
Figure 3. Schematic of a vitrification process (adapted from Ref. [15]).
The AVM process has been successfully used in France for 30 years, and, in Marcoule and La Hague, three vitrification installations were built based on this process. A similar plant was also built in Sellafield in the UK [17].
It has been estimated that vitrification of HLLW waste sludge into durable glass reduces volume by 77% when loading waste is about 28% (expressed as dry oxide mass). Vitrification of LLMW sludges and incinerator waste, in turn, reduces volume to 97% when loading waste is about 50–75%. This large reduction of the waste volume minimizes both long-term storage costs and the environmental danger [18].
The main advantages and shortcoming of the vitrification process are presented in Table 2.
| Advantages | Limitations | |
|---|---|---|
| Ex-Situ Vitrification (ESV) | In-Situ Vitrification (ISV) | |
| High volume reduction that arises to 90%. | The process requires high initial investment and operating costs. | |
| Final product is extremely chemically stable. | A state-of-the-art technology is designed to work at high temperatures, thus requiring highly qualified personnel. | |
| High efficiency, so fast decommissioning of waste. | Currently used types of glass do not accept large amounts of MoO3 and noble metals formed in the secondary waste streams. These compounds are poorly soluble in the borosilicate glasses, which reduces the amount of waste that can be loaded into material intended for vitrification and increases duration of the process and the operational costs. | |
| Applicability for different types of wet waste. | Use, storage, or disposal of the vitrified slag is required. Vitrification does not reduce radioactivity of the waste. Vitrified waste, being radioactively contaminated must be stored in facilities that protect the environment from radiation exposure. | Cannot be used with underground pipes, drums, and/or bricks if their amount exceeds 20% by weight. |
| The benefits mentioned above may significantly reduce cost waste savings. | Fragments of greater size than 60 mm should be removed waste intended for vitrification. | Heating the soil may cause the subsurface migration of radionuclides into clean areas. |
| Accumulation capability of volatile radionuclides in the melter off-gas system may cause danger to the staff and the environment. | Cannot be used in the area where exists large collection of flammable or explosive materials. | |
| Heat emission caused by cesium content in the vitreous form of waste should be estimated in order to not exceed the limits in force in the disposal site requirements. | The solidified material may obstruct future site use. | |
| If plutonium is being vitrified, it must be protected against robbery, as it can be recovered from glass. | Volatile organic compounds and certain radionuclides (e.g., Cs-137, Sr-90, and H-3) may be released as vapors. Control of the off-gases and the high voltage used, prevent potential safety risks. | |
| Despite reducing volume of the waste and mobility of radionuclides, it does not reduce radiation activity. So, protective barriers that limit exposure to radioactive emissions may still be required at some sites. | ||
Despite numerous limitations, vitrification is the most desirable method in places where large amounts of radioactive waste of similar composition are available, e.g., the HLW, resulting from operation of the nuclear power plants or the nuclear medicine laboratories.
2.3. Immobilization Radioactive Waste in the Synthetic Rock (Synroc)
In the 1970s, at Pennsylvania State University (USA), it was suggested that glass is relatively less thermodynamically stable than crystalline materials and that the use of synthetic minerals would be beneficial [21][22]. Minerals may have been stable for millions of years under conditions characteristic of a deep geological storage site [21][23]. So, at Australian National University (ANU), in 1978, a material was formulated, named Synroc, an abbreviation for synthetic rock. The name describes ceramic materials composed similarly to the titanate minerals. They were chosen because of their high chemical stability and pronounced ability to immobilize in the crystalline structure elements usually present in HLW. Minerals, proposed for Synroc, had been found in the environment and, over millennia under extremely difficult conditions, have demonstrated their ability to retain naturally occurring radioactive elements, such as uranium or thorium, among others. Different from borosilicate glass matrix, an amorphous solid solution without any crystalline form, Synroc ceramics incorporate radioactive waste in its crystalline structure.
Till now, a large number of the potential Synroc materials have demonstrated their high resistance against water leaching and, thus, demonstrated their potency in HLW immobilization. For example, V.M. Oversby, from Lawrence Livermore National Laboratory (USA), has proposed that waste from the Chernobyl Unit 4 reactor may be stored in a ceramic material that contains the mineral zirconolite (CaZrTi2O7; radionuclides in lattice: Zr, Rare Earths minor actinides [24]) with some addition of perovskite (CaTiO3; (Sr, RE, An) [25]), nepheline ((Na, K)AlSiO4; (Na, K, and Ca) [26]), and spinel (MgAl2O4; corrosion products [27]). It has been reported that waste loadings up to 50% of uranium can be achieved if pyrochlore (mineral, such as the zirconolite) is used [24].
Let us briefly present the thermochemical procedure for immobilization of radioactive waste proposed by the ANU and performed in Sandia [28]. In the first stage of three titanate minerals: hollandite, zirconolite, perovskite, and sometimes also rutile (titanium oxides), are combined into a slurry to which a certain quantity of HLLW is supplemented. This mixture is dried and calcined at 750 °C to obtain powder. Then, the material is inserted into a bellows-like stainless steel container and pressed at 1150–1200 °C. Finally, the resulting Synroc cylinder is loaded into the canister suitable for a long-term disposal. The scheme of the process using the Sandia method is presented in Figure 4.
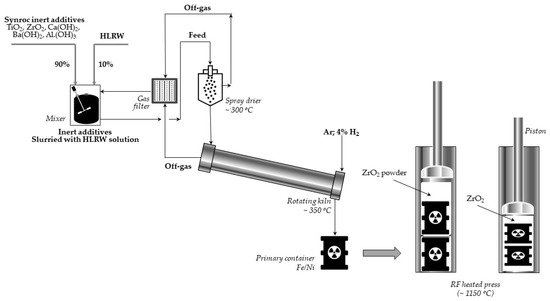
Figure 4. Scheme of the Synroc process using the Sandia method (adapted from Ref. [28]).
The problem with the Synroc method of disposal the radioactive waste, similarly to the vitrificated waste, arises during its long-term storage in deep underground storage facilities. Although the waste cannot be leached by different water streams, it remains strongly radioactive for a long time and can have a negative impact on the environment.
The leaching rate for Synroc depends on the element concerned. The highest values occur for Cs and Ba (1–2 × 10−7 g/cm2d; at 95 °C), while the lowest occurs for U (5 × 10−10 g/cm2d in the same conditions). It has been found also that the leaching rate significantly increases for nearly all the elements, while temperature of the process increases. Comparison of leaching rate for Synroc with data established for the borosilicate glass shows that, in the case of uranium, the process is 3000 times slower for Synroc than for glass [29].
The main advantages and shortcoming of the radioactive waste in the Synroc are presented in Table 3.
Table 3. Main advantages and limitations of immobilization the radioactive waste in the Synroc [19][20][30].
| Advantages | Shortcomings |
|---|---|
| The corrosion rate seems not to be the water flow-rate dependent. | Kinetic limitations and competition with caesium obviously affect the incorporation of strontium. |
| Big density of the Synroc materials allows to load about 60 wt% bigger amounts of waste into than the borosilicate glass. | As a ceramic nuclear waste form age, it sustains “damage” because of self-irradiation. |
| The water solubility of the main matrix component, is about 3–4 orders of magnitude lower than that of SiO2 used for vitrification. | During the aging of radioactive waste loaded into the Synroc matrix, self-irradiation damage can be sometimes observed. |
| High-temperature thermal stability of Synroc in hydrothermal environments allows for construction deeper disposal places in the earth’s crust. | Radioactive waste encased in the Synroc matrix may heat up internally because of the release of radioactive decay energy. |
| Naturally occurring, U- and Th-bearing phases which are isostructural with actinide bearing Synroc phases permit avoiding long-term alpha-decay damage effects in Synroc phases. |
This entry is adapted from the peer-reviewed paper 10.3390/en15010375
References
- Announcement of the Marshal of the Parliament of the Republic of Poland from 1 March 2021 on the Publication of the Consolidated Text of the Act—Atomic Law. J. Laws 2021, 623, 1–178. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20210000623/O/D20210623.pdf (accessed on 17 August 2020). (In Polish).
- Radioactive Waste Management: Collecting, Sorting, Treating, Conditioning, Storing and Disposing Safely Radioactive Waste. Available online: https://www.irsn.fr/EN/publications/thematic/Documents/irsn_booklet_radioactive_waste.pdf (accessed on 17 August 2020).
- Resolution No. 154 of the Council of Ministers of 21 October 2020, on Updating the “National Plan for Dealing with Radioactive Waste and Spent Nuclear Fuel”. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WMP20200001070/O/M20201070.pdf (accessed on 17 August 2020). (In Polish)
- Madaj, K. Radioactive waste in Poland. Nucl. Saf. Radiat. Prot. 2021, 2, 5–11.
- Hyatt, N.C.; Ojovan, M.I. Special Issue: Materials for Nuclear Waste Immobilization. Mater 2019, 12, 3611.
- Le, V.T.; Beamer, N.V.; Buckley, L.P. Experience with radioactive waste incineration at Chalk River Nuclear Laboratories. Waste Manag. 1989, 9, 67–72.
- Lefillatre, G.; Rodier, J.; Hullo, R.; Cudel, Y.; Rodi, L. Use of a Thin-Film Evaporator for Bitumen Coating of Radioactive Concentrates; Report CEA-R—3742; Commissariat a l’Energie Atomique: Chusclan, France, 1969.
- Ojovan, M.I.; Lee, W.E. An Introduction to Nuclear Waste Immobilisation, 2nd ed.; Elsevier: Kidlington, Oxford, UK, 2014.
- Chwaszczewski, S.; Klisińska, M. Spent Fuel and Radioactive Waste Management in Poland. In National Centre for Nuclear Research; Annual Report 2011; National Centre for Nuclear Research: Otwock-Świerk, Poland, 2011; p. 247.
- Dlouhý, Z. (Ed.) Fixation of Radioactive Concentrates, in Disposal of Radioactive Wastes. Stud. Environ. Sci. 1982, 15, 130–154.
- Zakharova, K.P.; Masanov, O.L. Bituminization of Liquid Radioactive Wastes. Safety Assessment and Operating Experience. At. Energy 2000, 89, 646–649.
- Lutze, W.; Ewing, R.C. Summary and Evaluation of Waste Forms in Radioactive Waste Forms for the Future; Lutze, W., Ewing, R.C., Eds.; North-Holland: Amsterdam, The Netherlands, 1988; pp. 699–740.
- Stoch, P.; Ciecinska, M.; Stoch, A. Thermal properties of phosphate glasses for salt waste immobilization. J. Therm. Anal. Calorim. 2014, 117, 197–204.
- Deptuła, A.; Miłkowska, M.; Łada, W.; Olczak, T.; Wawszczak, D.; Smolinski, T.; Brykala, M.; Chmielewski, A.G.; Zaza, F.; Goretta, K.C. Vitrification of nuclear wastes by complex sol-gel process. Adv. Mater. Res. 2012, 518–523, 3216–3220.
- Techniques for the Solidification of High Level Wastes, Technical Reports Series No. 176; IAEA: Vienna, Austria, 1977; Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/09/366/9366077.pdf?r=1&r=1 (accessed on 17 August 2020).
- Ojovan, M.A. Handbook of Advanced Radioactive Waste Conditioning Technologies; Woodhead Publishing: Cambridge, UK, 2011; p. 140.
- Riley, A.; Walker, S.; Gribble, N.R. Composition changes and future challenges for the Sellafield waste vitrification plant. Mater. Res. Soc. Symp. Proc. 2009, 1193, 267–273.
- Jantzen, C.M. Vitrification of Low Level and Mixed (Radioactive and Hazardous) Wastes: Lessons Learned from High Level Waste Vitrification; WSRC-MS—94-0334 Report; Westinghouse Savannah River Company: Aiken, CA, USA, 1994.
- Vitrification: The Workhorse of Nuclear Waste Management. Available online: https://mo-sci.com/vitrification-nuclear-waste-management (accessed on 17 August 2020).
- Stabilization/Solidification—Vitrification. Available online: http://www.cpeo.org/techtree/ttdescript/ssvit.htm (accessed on 17 August 2020).
- McCarthy, G.J. High-level waste ceramics: Materials considerations, process simulation, and product characterization. Nucl. Technol. 1977, 32, 92–105.
- Roy, R. Rational molecular engineering of ceramic materials. J. Am. Ceram. Soc. 1977, 60, 350–363.
- McCarthy, G.J.; White, W.B.; Pfoertsch, D.E. Synthesis of nuclear waste monazites, ideal actinide hosts for geologic disposal. Mater. Res. Bull. 1978, 13, 1239–1245.
- Oversby, V.M. Ceramic waste forms for fuel-containing masses at Chernobyl. In Proceedings of the Spectrum 94, Nuclear and Hazardous Waste Management International Topical Meeting, Atlanta, Georgia, USA, 14–18 August 1994; UCRL-JC—115171 Report. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:26006362 (accessed on 17 August 2020).
- Loiseau, P.; Touet, I.; Caurant, D.; Dextre, Y.; Fillet, C. Characterization of the Microstructure of Zirconolite-Based Glass-Ceramics. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/32/034/32034054.pdf (accessed on 17 August 2020).
- Singh, B.K.; Tomar, R.; Tomar, R.; Tomar, S.S. Sorption of homologues of radionuclides by synthetic ion exchanger. Microporous Mesoporous Mater 2011, 142, 629–640.
- Whitehead, N.E. A study of corrosion products using a cocktail of radionuclides. J. Radioanal. Nucl. Chem. 1994, 185, 45–55.
- Ringwood, A.E.; Oversby, V.M.; Kesson, S.E.; Sinclair, W.; Ware, N.; Hibberson, W.; Major, A. Immobilization of high-level nuclear reactor wastes in SYNROC: A current appraisal. Nucl. Chem. Waste Manag. 1981, 2, 287–305.
- Oversby, V.M.; Ringwood, A.E. Leaching studies on SYNROC at 95 °C and 200 °C. Radioact. Waste Manag. 1982, 2, 223–237.
- Ewing, R.C.; Lutze, W. High-level nuclear waste immobilization with ceramics. Ceram. Int. 1991, 17, 287–293.
This entry is offline, you can click here to edit this entry!
