Antibody ribosome display remains one of the most successful in vitro selection technologies for antibodies fifteen years after it was developed. The unique possibility of direct generation of whole proteins, particularly single-chain antibody fragments (scFvs), has facilitated the establishment of this technology as one of the foremost antibody production methods. Ribosome display has become a vital tool for efficient and low-cost production of antibodies for diagnostics due to its advantageous ability to screen large libraries and generate binders of high affinity. The remarkable flexibility of this method enables its applicability to various platforms.
- ribosome display
- • combinatorial libraries
- • biopanning
- • selection
- scFv
- diagnostics
- therapeutics
1. Introduction
2. In Vitro Ribosome Display
2.1. Selection of Antibodies by Panning
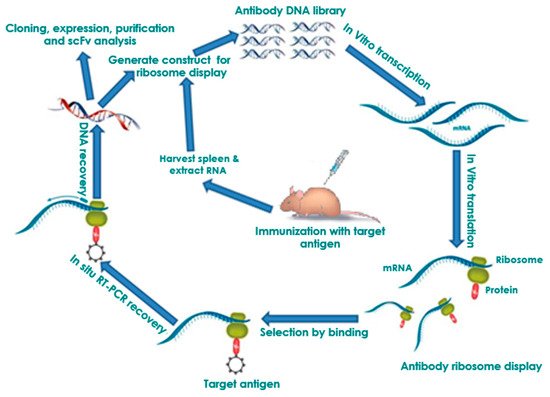
2.2. Affinity Maturation and Modification of Ribosome Display Antibodies
2.3. Ribosome Display Antibody Gene Libraries
3. Ribosome Display Technology in Disease Diagnostics and Control
3.1. Human Infectious Diseases
Significant spike mutations (D614G, E484K, K417N/T, N501Y, L452R, T478K) are found associated with different clinical consequences throughout the globe [4][110]. Scientists observed successful therapeutics from the significant clinical trials, including small antiviral molecules such as remdesivir or antibody-based therapeutics against SARS-CoV-2 [5][111]. Several antibodies have shown significant neutralization activity against the virus. Some antibodies have received EUA (Emergency Use Authorization) for the treatment of this virus. Most of the antibodies are designed against the S-glycoprotein of this virus. Therefore, any S-glycoprotein mutations can trigger the antibody escapes/antibody resistance in SARS-CoV-2 variants and hinder the antibody-based therapeutic strategies against the virus [4][110]. To avoid escape mutants, ribosome display technology could possibly be applied to target highly conserved epitopes to produce neutralizing scFv antibody cocktails targeting simultaneously non-RBD and RBD epitopes. Neutralizing antibody cocktails against SARS-CoV-2 would help in the development of diagnostics and treatment for COVID-19.
3.2. Cancer
3.3. Acquired Immunodeficiency Syndrome (AIDS)
3.4. Plant Disease: Pierce’s Disease
3.5. Pain
scFv antibodies are opening a new era of therapeutics, pharmacology, and pathophysiology research [6][153]. These technologies have overcome previous challenges of providing therapeutic applications for G-protein-coupled receptors (GPCRs). More importantly, these small, brain penetrant antibodies are praised as having promising biotherapeutic applications for the nervous and immune systems, now recognized as interactive in chronic pain. scFvs are being investigated as therapeutics for arthritis, Creutzfeldt-Jakob, and Huntington’s disease due to their solubility, small size, and ability to cross the blood-brain barrier compared to mAbs available for migraine (Galcanezumab, Erenumab) [7][8][9][154-156]. Despite the popularity of scFvs generated by ribosome display for chemotherapy, obtaining high-affinity scFvs from ribosome display libraries remains a challenging task [10][157].
Chronic pain frequently evokes anxiety, depression, disability, and diminishes quality of life. It is known that cholecystokinin (CCK) evokes anxiety/panic attacks in healthy subjects depending on dosage, and it is 103 times more abundant than any other neuropeptide in the nervous system. Selective antagonists of the CCKB receptor (CCKBR) enhance morphine analgesia and prevent tolerance without worsening respiratory depression in non-human primates or side effects other than orthostatic dizziness in placebo-controlled trials. We have generated a scFv biological that targets mouse CCKBR using ribosome display [11][158]. The small CCKBR scFv is ~1/6 the size of a monoclonal antibody thus can access the CCKBR biodistribution to positively impact pain circuitry neurons. Its high affinity binding permanently reverses chronic pain-, cognitive-, anxiety-, and depression-related behaviors.
A serious consequence of nerve injury pain or “neuropathic pain” is the transition to chronic pain that remains a significant clinical challenge with a treatment response rate of only 11% [12][13][159, 160]. While decades of study have been devoted to acute “nociceptive” mechanisms, it is clear that complex, multifactorial mechanisms are responsible for maintaining neuropathic pain long term, referred to as the “chronification” of pain. Current understanding is pain chronification causes physiological, molecular, epigenetic, and brain circuitry changes. While most studies are done in acute pain models, we utilize clinically relevant models of chronic neuropathic pain and find significantly reduced pain related behaviors after a single treatment with our scFv antibody targeting the P2X4 receptor (P2X4R) using ribosome display [14][161]. P2X4R upregulation occurs in chronic pain, attributed to microglia in males and to T cells in females [15][162]. These data provide support for pursuit of P2X4R scFvs as translational therapy for pain relief. We hope to develop non-opioid therapies to treat chronic pain using small protein, brain penetrant, single chain Fragment variable (scFv) antibody therapies. These have the potential to reverse chronic neuropathic pain, associated pain-related behaviors and depression.
This entry is adapted from the peer-reviewed paper 10.3390/antib9030028
References
- Chiranjib Chakraborty; Manojit Bhattacharya; Ashish Ranjan Sharma; Present variants of concern and variants of interest of severe acute respiratory syndrome coronavirus 2: Their significant mutations in S‐glycoprotein, infectivity, re‐infectivity, immune escape and vaccines activity. Reviews in Medical Virology 2021, 0, e2270, 10.1002/rmv.2270.
- Aleem, A.; AB, A.S.; Slenker A.K.. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19); In: StatPearls, Eds.; StatPearls Publishing : Treasure Island (FL), 2022; pp. PMID: 34033342.
- William T. Harvey; Alessandro M. Carabelli; Ben Jackson; Ravindra K. Gupta; Emma C. Thomson; Ewan M. Harrison; Catherine Ludden; Richard Reeve; Andrew Rambaut; Sharon J. Peacock; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nature Reviews Microbiology 2021, 19, 409-424, 10.1038/s41579-021-00573-0.
- Kaiming Tao; Philip L. Tzou; Janin Nouhin; Ravindra K. Gupta; Tulio de Oliveira; Sergei L. Kosakovsky Pond; Daniela Fera; Robert W. Shafer; The biological and clinical significance of emerging SARS-CoV-2 variants. Nature Reviews Genetics 2021, 22, 757-773, 10.1038/s41576-021-00408-x.
- Chiranjib Chakraborty; Ashish Ranjan Sharma; Manojit Bhattacharya; Govindasamy Agoramoorthy; Sang-Soo Lee; The Drug Repurposing for COVID-19 Clinical Trials Provide Very Effective Therapeutic Combinations: Lessons Learned From Major Clinical Studies. Frontiers in Pharmacology 2021, 12, 704205, 10.3389/fphar.2021.704205.
- Mohammed Akli Ayoub; Pascale Crépieux; Markus Koglin; Marc Parmentier; Jean-Philippe Pin; Anne Poupon; Eric Reiter; Martine Smit; Jan Steyaert; Hervé Watier; et al. Antibodies targeting G protein-coupled receptors: Recent advances and therapeutic challenges. Platform development for expression and purification of stable isotope labeled monoclonal antibodies in Escherichia coli 2017, 9, 735-741, 10.1080/19420862.2017.1325052.
- David C. Butler; Julie A. McLear; Anne Messer; Engineered antibody therapies to counteract mutant huntingtin and related toxic intracellular proteins. Progress in Neurobiology 2011, 97, 190-204, 10.1016/j.pneurobio.2011.11.004.
- Nives Škrlj; Marko Dolinar; New engineered antibodies against prions.. Bioengineered 2013, 5, 10-4, 10.4161/bioe.26069.
- Alessandro Angelini; Yoshishige Miyabe; Daniel Newsted; Byron H. Kwan; Chie Miyabe; Ryan L. Kelly; Misha N. Jamy; Andrew D. Luster; K. Dane Wittrup; Directed evolution of broadly crossreactive chemokine-blocking antibodies efficacious in arthritis. Nature Communications 2018, 9, 1461, 10.1038/s41467-018-03687-x.
- Zuhaida Asra Ahmad; Swee Keong Yeap; Abdul Manaf Ali; Wan Yong Ho; Noorjahan Banu Mohamed Alitheen; Muhajir Hamid; scFv Antibody: Principles and Clinical Application. Clinical and Developmental Immunology 2012, 2012, 1-15, 10.1155/2012/980250.
- K.N. Westlund; M.A. Montera; A.E. Goins; S.R.A. Alles; M. Afaghpour-Becklund; R. Bartel; R. Durvasula; A. Kunamneni; Single-chain Fragment variable antibody targeting cholecystokinin-B receptor for pain reduction. Neurobiology of Pain 2021, 10, 100067, 10.1016/j.ynpai.2021.100067.
- Yaron Haviv; Yehuda Zadik; Yair Sharav; Rafael Benoliel; Painful Traumatic Trigeminal Neuropathy: An Open Study on the Pharmacotherapeutic Response to Stepped Treatment. Journal of Oral & Facial Pain and Headache 2013, 28, 52-60, 10.11607/jop.1154.
- Lene Baad-Hansen; Rafael Benoliel; Neuropathic orofacial pain: Facts and fiction. Cephalalgia 2017, 37, 670-679, 10.1177/0333102417706310.
- Karin N. Westlund; Marena A. Montera; Aleyah E. Goins; Sascha R. A. Alles; Nikita Suri; Sabrina L. McIlwrath; Robyn Bartel; Ravi V. Durvasula; Adinarayana Kunamneni; Single-Dose P2 X4R Single-Chain Fragment Variable Antibody Permanently Reverses Chronic Pain in Male Mice. International Journal of Molecular Sciences 2021, 22, 13612, 10.3390/ijms222413612.
- Robert E. Sorge; Josiane Mapplebeck; Sarah Rosen; Simon Beggs; Sarah Taves; Jessica K. Alexander; Loren Martin; Jean-Sebastien Austin; Susana G. Sotocinal; Di Chen; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature Neuroscience 2015, 18, 1081-1083, 10.1038/nn.4053.
- He, M.; Taussig, M.J. Selection of recombinant antibodies by eukaryotic ribosome display. Methods Mol. Biol. 2008, 484, 193–205.
- Stafford, R.; Matsumoto, M.; Yin, G.; Cai, Q.; Fung, J.J.; Stephenson, H.; Gill, A.; You, M.; Lin, S.-H.; Wang, W.; et al. In vitro Fab display: A cell-free system for IgG discovery. Protein Eng. Des. Sel. Peds 2014, 27.
- Mattheakis, L.C.; Dias, J.M.; Dower, W.J. Cell-free synthesis of peptide libraries displayed on polysomes. Methods Enzym. 1996, 267, 195–207.
- Gersuk, G.M.; Corey, M.J.; Corey, E.; Stray, J.E.; Kawasaki, G.H.; Vessella, R.L. High-affinity peptide ligands to prostate-specific antigen identified by polysome selection. Biochem. Biophys. Res. Commun. 1997, 232, 578–582.
- Hanes, J.; Jermutus, L.; Weber-Bornhauser, S.; Bosshard, H.R.; Pluckthun, A. Ribosome display efficiently selects and evolves high-affinity antibodies in vitro from immune libraries. Proc. Natl. Acad. Sci. USA 1998, 95, 14130–14135.
- Hanes, J.; Jermutus, L.; Schaffitzel, C.; Pluckthun, A. Comparison of Escherichia coli and rabbit reticulocyte ribosome display systems. Febs Lett. 1999, 450, 105–110.
- Lee, M.S.; Kwon, M.H.; Kim, K.H.; Shin, H.J.; Park, S.; Kim, H.I. Selection of scFvs specific for HBV DNA polymerase using ribosome display. J. Immunol. Methods 2004, 284, 147–157.
- He, M.; Taussig, M.J. Ribosome display of antibodies: Expression, specificity and recovery in a eukaryotic system. J. Immunol. Methods 2005, 297, 73–82.
- Kim, J.M.; Shin, H.J.; Kim, K.; Lee, M.S. A pseudoknot improves selection efficiency in ribosome display. Mol. Biotechnol. 2007, 36, 32–37.
- Qi, Y.; Wu, C.; Zhang, S.; Wang, Z.; Huang, S.; Dai, L.; Wang, S.; Xia, L.; Wen, K.; Cao, X.; et al. Selection of anti-sulfadimidine specific ScFvs from a hybridoma cell by eukaryotic ribosome display. PLoS ONE 2009, 4, e6427.
- Kastelic, D.; He, M. Ribosome display and screening for protein therapeutics. Methods Mol. Biol. 2012, 899, 61–72.
- Edwards, B.M.; He, M. Evolution of antibodies in vitro by ribosome display. Methods Mol. Biol. 2012, 907, 281–292.
- Douthwaite, J.A. Eukaryotic ribosome display selection using rabbit reticulocyte lysate. Methods Mol. Biol. 2012, 805, 45–57.
- Tang, J.; Wang, L.; Markiv, A.; Jeffs, S.A.; Dreja, H.; McKnight, A.; He, M.; Kang, A.S. Accessing of recombinant human monoclonal antibodies from patient libraries by eukaryotic ribosome display. Hum. Antibodies 2012, 21, 1–11.
- Parmley, S.F.; Smith, G.P. Antibody-selectable filamentous fd phage vectors: Affinity purification of target genes. Gene 1988, 73, 305–318.
- Bittner, M.; Kupferer, P.; Morris, C.F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal. Biochem. 1980, 102, 459–471.
- Hawlisch, H.; Muller, M.; Frank, R.; Bautsch, W.; Klos, A.; Kohl, J. Site-specific anti-C3a receptor single-chain antibodies selected by differential panning on cellulose sheets. Anal. Biochem. 2001, 293, 142–145.
- Moghaddam, A.; Borgen, T.; Stacy, J.; Kausmally, L.; Simonsen, B.; Marvik, O.J.; Brekke, O.H.; Braunagel, M. Identification of scFv antibody fragments that specifically recognise the heroin metabolite 6-monoacetylmorphine but not morphine. J. Immunol. Methods 2003, 280, 139–155.
- Breitling, F.; Dubel, S.; Seehaus, T.; Klewinghaus, I.; Little, M. A surface expression vector for antibody screening. Gene 1991, 104, 147–153.
- Hust, M.; Maiss, E.; Jacobsen, H.J.; Reinard, T. The production of a genus-specific recombinant antibody (scFv) using a recombinant potyvirus protease. J. Virol. Methods 2002, 106, 225–233.
- Barbas, C.F., 3rd; Kang, A.S.; Lerner, R.A.; Benkovic, S.J. Assembly of combinatorial antibody libraries on phage surfaces: The gene III site. Proc. Natl. Acad. Sci. USA 1991, 88, 7978–7982.
- Sanna, P.P.; Williamson, R.A.; De Logu, A.; Bloom, F.E.; Burton, D.R. Directed selection of recombinant human monoclonal antibodies to herpes simplex virus glycoproteins from phage display libraries. Proc. Natl. Acad. Sci. USA 1995, 92, 6439–6443.
- Winter, G.; Milstein, C. Man-made antibodies. Nature 1991, 349, 293–299.
- Hoogenboom, H.R. Selecting and screening recombinant antibody libraries. Nat Biotechnol 2005, 23, 1105–1116.
- Hust, M.; Frenzel, A.; Schirrmann, T.; Dubel, S. Selection of recombinant antibodies from antibody gene libraries. Methods Mol Biol 2014, 1101, 305–320.
- Hust, M.; Dubel, S.; Schirrmann, T. Selection of recombinant antibodies from antibody gene libraries. Methods Mol. Biol. 2007, 408, 243–255.
- Hallborn, J.; Carlsson, R. Automated screening procedure for high-throughput generation of antibody fragments. Biotechniques 2002, 33 (Suppl. S6), 30–37.
- Konthur, Z.; Hust, M.; Dubel, S. Perspectives for systematic in vitro antibody generation. Gene 2005, 364, 19–29.
- Schirrmann, T.; Al-Halabi, L.; Dubel, S.; Hust, M. Production systems for recombinant antibodies. Front Biosci. 2008, 13, 4576–4594.
- Hust, M.; Meyer, T.; Voedisch, B.; Rulker, T.; Thie, H.; El-Ghezal, A.; Kirsch, M.I.; Schutte, M.; Helmsing, S.; Meier, D.; et al. A human scFv antibody generation pipeline for proteome research. J. Biotechnol. 2011, 152, 159–170.
- Groves, M.A.; Nickson, A.A. Affinity maturation of phage display antibody populations using ribosome display. Methods Mol. Biol. 2012, 805, 163–1690.
- Kobayashi, N.; Oyama, H.; Kato, Y.; Goto, J.; Soderlind, E.; Borrebaeck, C.A. Two-step in vitro antibody affinity maturation enables estradiol-17beta assays with more than 10-fold higher sensitivity. Anal. Chem. 2010, 82, 1027–1038.
- Finlay, W.J.; Cunningham, O.; Lambert, M.A.; Darmanin-Sheehan, A.; Liu, X.; Fennell, B.J.; Mahon, C.M.; Cummins, E.; Wade, J.M.; O’Sullivan, C.M.; et al. Affinity maturation of a humanized rat antibody for anti-RAGE therapy: Comprehensive mutagenesis reveals a high level of mutational plasticity both inside and outside the complementarity-determining regions. J. Mol. Biol. 2009, 388, 541–558.
- Lipovsek, D.; Pluckthun, A. In-vitro protein evolution by ribosome display and mRNA display. J. Immunol. Methods 2004, 290, 51–67.
- Hanes, J.; Schaffitzel, C.; Knappik, A.; Pluckthun, A. Picomolar affinity antibodies from a fully synthetic naive library selected and evolved by ribosome display. Nat. Biotechnol. 2000, 18, 1287–1292.
- Schaffitzel, C.; Berger, I.; Postberg, J.; Hanes, J.; Lipps, H.J.; Pluckthun, A. In vitro generated antibodies specific for telomeric guanine-quadruplex DNA react with Stylonychia lemnae macronuclei. Proc. Natl. Acad. Sci. USA 2001, 98, 8572–8577.
- Jermutus, L.; Honegger, A.; Schwesinger, F.; Hanes, J.; Pluckthun, A. Tailoring in vitro evolution for protein affinity or stability. Proc. Natl. Acad. Sci. USA 2001, 98, 75–80.
- Levin, A.M.; Weiss, G.A. Optimizing the affinity and specificity of proteins with molecular display. Mol. Biosyst. 2006, 2, 49–57.
- Dreier, B.; Pluckthun, A. Rapid selection of high-affinity binders using ribosome display. Methods Mol. Biol. 2012, 805, 261–286.
- Lewis, L.; Lloyd, C. Optimisation of antibody affinity by ribosome display using error-prone or site-directed mutagenesis. Methods Mol. Biol. 2012, 805, 139–161.
- Zahnd, C.; Spinelli, S.; Luginbuhl, B.; Amstutz, P.; Cambillau, C.; Pluckthun, A. Directed in vitro evolution and crystallographic analysis of a peptide-binding single chain antibody fragment (scFv) with low picomolar affinity. J. Biol. Chem. 2004, 279, 18870–18877.
- Chin, S.E.; Ferraro, F.; Groves, M.; Liang, M.; Vaughan, T.J.; Dobson, C.L. Isolation of high affinity, neutralizing anti-idiotype antibodies by phage and ribosome display for application in immunogenicity and pharmacokinetic analyses. J. Immunol. Methods 2015, 416, 49–58.
- Groves, M.A.; Amanuel, L.; Campbell, J.I.; Rees, D.G.; Sridharan, S.; Finch, D.K.; Lowe, D.C.; Vaughan, T.J. Antibody VH and VL recombination using phage and ribosome display technologies reveals distinct structural routes to affinity improvements with VH-VL interface residues providing important structural diversity. MAbs 2014, 6, 236–245.
- Hu, D.; Tateno, H.; Hirabayashi, J. Directed evolution of lectins by an improved error-prone PCR and ribosome display method. Methods Mol. Biol. 2014, 1200, 527–538.
- Kanamori, T.; Fujino, Y.; Ueda, T. PURE ribosome display and its application in antibody technology. Biochim. Biophys. Acta 2014, 1844, 1925–1932.
- Darmanin-Sheehan, A.; Finlay, W.J.; Cunningham, O.; Fennell, B.J. Molecular scanning: Combining random mutagenesis, ribosome display, and bioinformatic analysis for protein engineering. Methods Mol. Biol. 2012, 907, 487–503.
- Lei, L. Identification of candidate vaccine genes using ribosome display. Methods Mol. Biol. 2012, 805, 299–314.
- Groves, M.A.; Osbourn, J.K. Applications of ribosome display to antibody drug discovery. Expert Opin. Biol. 2005, 5, 125–135.
- Chodorge, M.; Fourage, L.; Ravot, G.; Jermutus, L.; Minter, R. In vitro DNA recombination by L-Shuffling during ribosome display affinity maturation of an anti-Fas antibody increases the population of improved variants. Protein Eng. Des. Sel. Peds 2008, 21, 343–351.
- Heyduk, E.; Heyduk, T. Ribosome display enhanced by next generation sequencing: A tool to identify antibody-specific peptide ligands. Anal. Biochem. 2014, 464, 73–82.
- Li, R.; Kang, G.; Hu, M.; Huang, H. Ribosome Display: A Potent Display Technology used for Selecting and Evolving Specific Binders with Desired Properties. Mol. Biotechnol. 2019, 61, 60–71.
- Ryabova, L.A.; Desplancq, D.; Spirin, A.S.; Plückthun, A. Functional antibody production using cell-free translation: Effects of protein disulfide isomerase and chaperones. Nat. Biotechnol. 1997, 15, 79–84.
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature 1990, 348, 552–554.
- Glockshuber, R.; Malia, M.; Pfitzinger, I.; Plueckthun, A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry 1990, 29, 1362–1367.
- Groves, M.; Lane, S.; Douthwaite, J.; Lowne, D.; Rees, D.G.; Edwards, B.; Jackson, R.H. Affinity maturation of phage display antibody populations using ribosome display. J. Immunol. Methods 2006, 313, 129–139.
- Sun, Y.; Ning, B.; Liu, M.; Gao, X.; Fan, X.; Liu, J.; Gao, Z. Selection of diethylstilbestrol-specific single-chain antibodies from a non-immunized mouse ribosome display library. PLoS ONE 2012, 7, e33186.
- Whiteaker, J.R.; Zhao, L.; Frisch, C.; Ylera, F.; Harth, S.; Knappik, A.; Paulovich, A.G. High-affinity recombinant antibody fragments (Fabs) can be applied in peptide enrichment immuno-MRM assays. J. Proteome Res. 2014, 13, 2187–2196.
- Ylera, F.; Harth, S.; Waldherr, D.; Frisch, C.; Knappik, A. Off-rate screening for selection of high-affinity anti-drug antibodies. Anal. Biochem. 2013, 441, 208–213.
- Binz, H.K.; Stumpp, M.T.; Forrer, P.; Amstutz, P.; Pluckthun, A. Designing repeat proteins: Well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J. Mol. Biol. 2003, 332, 489–503.
- Binz, H.K.; Amstutz, P.; Kohl, A.; Stumpp, M.T.; Briand, C.; Forrer, P.; Grutter, M.G.; Pluckthun, A. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat. Biotechnol. 2004, 22, 575–582.
- Dreier, B.; Pluckthun, A. Ribosome display: A technology for selecting and evolving proteins from large libraries. Methods Mol. Biol. 2011, 687, 283–306.
- Schilling, J.; Schoppe, J.; Pluckthun, A. From DARPins to LoopDARPins: Novel LoopDARPin design allows the selection of low picomolar binders in a single round of ribosome display. J. Mol. Biol. 2014, 426, 691–721.
- Pluckthun, A. Ribosome display: A perspective. Methods Mol. Biol. 2012, 805, 3–28.
- Stefan, N.; Martin-Killias, P.; Wyss-Stoeckle, S.; Honegger, A.; Zangemeister-Wittke, U.; Pluckthun, A. DARPins recognizing the tumor-associated antigen EpCAM selected by phage and ribosome display and engineered for multivalency. J. Mol. Biol. 2011, 413, 826–843.
- Schilling, J.; Schoppe, J.; Sauer, E.; Pluckthun, A. Co-crystallization with conformation-specific designed ankyrin repeat proteins explains the conformational flexibility of BCL-W. J. Mol. Biol. 2014, 426, 2346–2362.
- Scholz, O.; Hansen, S.; Pluckthun, A. G-quadruplexes are specifically recognized and distinguished by selected designed ankyrin repeat proteins. Nucleic Acids Res. 2014, 42, 9182–9194.
- Brauchle, M.; Hansen, S.; Caussinus, E.; Lenard, A.; Ochoa-Espinosa, A.; Scholz, O.; Sprecher, S.G.; Pluckthun, A.; Affolter, M. Protein interference applications in cellular and developmental biology using DARPins that recognize GFP and mCherry. Biol. Open 2014, 3, 1252–1261.
- Tamaskovic, R.; Simon, M.; Stefan, N.; Schwill, M.; Pluckthun, A. Designed ankyrin repeat proteins (DARPins) from research to therapy. Methods Enzym. 2012, 503, 101–134.
- Wetzel, S.K.; Ewald, C.; Settanni, G.; Jurt, S.; Pluckthun, A.; Zerbe, O. Residue-resolved stability of full-consensus ankyrin repeat proteins probed by NMR. J. Mol. Biol. 2010, 402, 241–258.
- Wetzel, S.K.; Settanni, G.; Kenig, M.; Binz, H.K.; Pluckthun, A. Folding and unfolding mechanism of highly stable full-consensus ankyrin repeat proteins. J. Mol. Biol. 2008, 376, 241–257.
- Schweizer, A.; Roschitzki-Voser, H.; Amstutz, P.; Briand, C.; Gulotti-Georgieva, M.; Prenosil, E.; Binz, H.K.; Capitani, G.; Baici, A.; Pluckthun, A.; et al. Inhibition of caspase-2 by a designed ankyrin repeat protein: Specificity, structure, and inhibition mechanism. Structure 2007, 15, 625–636.
- Zahnd, C.; Wyler, E.; Schwenk, J.M.; Steiner, D.; Lawrence, M.C.; McKern, N.M.; Pecorari, F.; Ward, C.W.; Joos, T.O.; Pluckthun, A. A designed ankyrin repeat protein evolved to picomolar affinity to Her2. J. Mol. Biol. 2007, 369, 1015–1028.
- Zahnd, C.; Pecorari, F.; Straumann, N.; Wyler, E.; Pluckthun, A. Selection and characterization of Her2 binding-designed ankyrin repeat proteins. J. Biol. Chem. 2006, 281, 35167–35175.
- Amstutz, P.; Koch, H.; Binz, H.K.; Deuber, S.A.; Pluckthun, A. Rapid selection of specific MAP kinase-binders from designed ankyrin repeat protein libraries. Protein Eng. Des. Sel. Peds 2006, 19, 219–229.
- Amstutz, P.; Binz, H.K.; Parizek, P.; Stumpp, M.T.; Kohl, A.; Grutter, M.G.; Forrer, P.; Pluckthun, A. Intracellular kinase inhibitors selected from combinatorial libraries of designed ankyrin repeat proteins. J. Biol. Chem. 2005, 280, 24715–24722.
- Dreier, B.; Mikheeva, G.; Belousova, N.; Parizek, P.; Boczek, E.; Jelesarov, I.; Forrer, P.; Pluckthun, A.; Krasnykh, V. Her2-specific multivalent adapters confer designed tropism to adenovirus for gene targeting. J. Mol. Biol. 2011, 405, 410–426.
- Veesler, D.; Dreier, B.; Blangy, S.; Lichiere, J.; Tremblay, D.; Moineau, S.; Spinelli, S.; Tegoni, M.; Pluckthun, A.; Campanacci, V.; et al. Crystal structure and function of a DARPin neutralizing inhibitor of lactococcal phage TP901–1: Comparison of DARPin and camelid VHH binding mode. J. Bio.l Chem. 2009, 284, 30718–30726.
- Milovnik, P.; Ferrari, D.; Sarkar, C.A.; Pluckthun, A. Selection and characterization of DARPins specific for the neurotensin receptor 1. Protein Eng. Des. Sel. Peds 2009, 22, 357–366.
- Scholz, O.; Henssler, E.M.; Bail, J.; Schubert, P.; Bogdanska-Urbaniak, J.; Sopp, S.; Reich, M.; Wisshak, S.; Kostner, M.; Bertram, R.; et al. Activity reversal of Tet repressor caused by single amino acid exchanges. Mol. Microbiol. 2004, 53, 777–789.
- Yau, K.Y.; Dubuc, G.; Li, S.; Hirama, T.; Mackenzie, C.R.; Jermutus, L.; Hall, J.C.; Tanha, J. Affinity maturation of a V(H)H by mutational hotspot randomization. J. Immunol. Methods 2005, 297, 213–224.
- Perruchini, C.; Pecorari, F.; Bourgeois, J.P.; Duyckaerts, C.; Rougeon, F.; Lafaye, P. Llama VHH antibody fragments against GFAP: Better diffusion in fixed tissues than classical monoclonal antibodies. Acta Neuropathol. 2009, 118, 685–695.
- WHO. Ebola Virus Disease (Fact Sheets). Archived 30th May 2019. Available online: https://www.who.int/en/news-room/fact-sheets/detail/ebola-virus-disease (accessed on 4 February 2020).
- Kunamneni, A.; Clarke, E.C.; Ye, C.; Bradfute, S.B.; Durvasula, R. Generation and Selection of a Panel of Pan-Filovirus Single-Chain Antibodies using Cell-Free Ribosome Display. Am. J. Trop. Med. Hyg. 2019, 101, 198–206.
- Malone, R.W.; Homan, J.; Callahan, M.V.; Glasspool-Malone, J.; Damodaran, L.; Schneider Ade, B.; Zimler, R.; Talton, J.; Cobb, R.R.; Ruzic, I.; et al. Zika Virus: Medical Countermeasure Development Challenges. Plos Negl. Trop. Dis. 2016, 10, e0004530.
- WHO. Zika Virus (Fact Sheets). Archived 20th July 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/zika-virus (accessed on 4 February 2020).
- Kunamneni, A.; Ye, C.; Bradfute, S.B.; Durvasula, R. Ribosome display for the rapid generation of high-affinity Zika-neutralizing single-chain antibodies. PLoS ONE 2018, 13, e0205743.
- WHO. Tuberculosis. Archived 24th March, 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 12 May 2020).
- Getahun, H.; Harrington, M.; O’Brien, R.; Nunn, P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: Informing urgent policy changes. Lancet 2007, 369, 2042–2049.
- Ahangarzadeh, S.; Bandehpour, M.; Kazemi, B. Selection of single-chain variable fragments specific for Mycobacterium tuberculosis ESAT-6 antigen using ribosome display. Iran J. Basic Med. Sci. 2017, 20, 327–333.
- WHO. Q&A on Coronaviruses (COVID-19). Archived 17th April, 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses (accessed on 12 May 2020).
- (WHO), W.H.O. Coronavirus disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 12 May 2020).
- Schaffitzel, C.; Hanes, J.; Jermutus, L.; Pluckthun, A. Ribosome display: An in vitro method for selection and evolution of antibodies from libraries. J. Immunol. Methods 1999, 231, 119–135.
- Huang, S.; Feng, L.; An, G.; Zhang, X.; Zhao, Z.; Han, R.; Lei, F.; Zhang, Y.; Luo, A.; Jing, X.; et al. Ribosome display and selection of single-chain variable fragments effectively inhibit growth and progression of microspheres in vitro and in vivo. Cancer Sci. 2018, 109, 1503–1512.
- Center for Disease Control and Prevention (CDC), About HIV/AIDS. Archived 2nd December, 2019. Available online: https://www.cdc.gov/hiv/basics/whatishiv.html (accessed on 17 May 2020).
- WHO. HIV/AIDS. Archived 17th April, 2020. Available online: https://www.who.int/health-topics/hiv-aids/#tab=tab_1 (accessed on 17 May 2020).
- Burton, D.R.; Weiss, R.A. AIDS/HIV. A boost for HIV vaccine design. Science 2010, 329, 770–773.
- Scheid, J.F.; Mouquet, H.; Ueberheide, B.; Diskin, R.; Klein, F.; Oliveira, T.; Pietzsch, J.; Fenyö, D.; Abadir, A.; Velinzon, K.; et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 2011, 333, 1633–1637.
- Walker, L.M.; Huber, M.; Doores, K.J.; Falkowska, E.; Pejchal, R.; Julien, J.P.; Wang, S.K.; Ramos, A.; Chan-Hui, P.Y.; Moyle, M.; et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011, 477, 466–470.
- Davis, M.J.; Purcell, A.H.; Thomson, S.V. Pierce’s disease of grapevines: Isolation of the causal bacterium. Science 1978, 199, 75–77.
- Morano, L.D.; Bextine, B.R.; Garcia, D.A.; Maddox, S.V.; Gunawan, S.; Vitovsky, N.J.; Black, M.C. Initial genetic analysis of Xylella fastidiosa in Texas. Curr. Microbiol. 2008, 56, 346–351.
- Ramirez, J.L.; Lacava, P.T.; Miller, T.A. Detection of the bacterium, Xylella fastidiosa, in saliva of glassy-winged sharpshooter, Homalodisca vitripennis. J. Insect Sci. 2008, 8, 1–7.
- Myers, A.L.; Sutton, T.B.; Abad, J.A.; Kennedy, G.G. Pierce’s Disease of Grapevines: Identification of the Primary Vectors in North Carolina. Phytopathology 2007, 97, 1440–1450.
- Jackson, B.C.; Blua, M.J.; Bextine, B. Impact of duration versus frequency of probing by Homalodisca vitripennis (Hemiptera: Cicadellidae) on inoculation of Xylella fastidiosa. J. Econ. Entomol. 2008, 101, 1122–1126.
- Hendson, M.; Purcell, A.H.; Chen, D.; Smart, C.; Guilhabert, M.; Kirkpatrick, B. Genetic diversity of Pierce’s disease strains and other pathotypes of Xylella fastidiosa. Appl. Environ. Microbiol. 2001, 67, 895–903.
- Simpson, A.J.; Reinach, F.C.; Arruda, P.; Abreu, F.A.; Acencio, M.; Alvarenga, R.; Alves, L.M.; Araya, J.E.; Baia, G.S.; Baptista, C.S.; et al. The genome sequence of the plant pathogen Xylella fastidiosa. The Xylella fastidiosa Consortium of the Organization for Nucleotide Sequencing and Analysis. Nature 2000, 406, 151–159.
- Retchless, A.; Labroussaa, F.; Shapiro, L.; Stenger, D.; Lindow, S.; Almeida, R. Genomic Insights into Xylella Fastidiosa Interactions with Plant and Insect Hosts; Springer: Berlin, Germany, 2014; pp. 177–202.
- Caserta, R.; Takita, M.A.; Targon, M.L.; Rosselli-Murai, L.K.; de Souza, A.P.; Peroni, L.; Stach-Machado, D.R.; Andrade, A.; Labate, C.A.; Kitajima, E.W.; et al. Expression of Xylella fastidiosa fimbrial and afimbrial proteins during biofilm formation. Appl. Environ. Microbiol. 2010, 76, 4250–4259.
- Fjellbirkeland, A.; Bemanian, V.; McDonald, I.R.; Murrell, J.C.; Jensen, H.B. Molecular analysis of an outer membrane protein, MopB, of Methylococcus capsulatus (Bath) and structural comparisons with proteins of the OmpA family. Arch. Microbiol. 2000, 173, 346–351.
- Voegel, T.M.; Warren, J.G.; Matsumoto, A.; Igo, M.M.; Kirkpatrick, B.C. Localization and characterization of Xylella fastidiosa haemagglutinin adhesins. Microbiology 2010, 156 Pt 7, 2172–2179.
- Pierce, B.K.; Voegel, T.; Kirkpatrick, B.C. The Xylella fastidiosa PD1063 protein is secreted in association with outer membrane vesicles. PLoS ONE 2014, 9, e113504.
- Bishop, R.E. Structural biology of membrane-intrinsic beta-barrel enzymes: Sentinels of the bacterial outer membrane. Biochim. Et Biophys. Acta 2008, 1778, 1881–1896.
- Koebnik, R.; Locher, K.P.; Van Gelder, P. Structure and function of bacterial outer membrane proteins: Barrels in a nutshell. Mol. Microbiol. 2000, 37, 239–253.
- Kostakioti, M.; Newman, C.L.; Thanassi, D.G.; Stathopoulos, C. Mechanisms of protein export across the bacterial outer membrane. J. Bacteriol. 2005, 187, 4306–4314.
- Chen, Y.Y.; Wu, C.H.; Lin, J.W.; Weng, S.F.; Tseng, Y.H. Mutation of the gene encoding a major outer-membrane protein in Xanthomonas campestris pv. campestris causes pleiotropic effects, including loss of pathogenicity. Microbiology 2010, 156 Pt 9, 2842–2854.
- Gotoh, N.; Wakebe, H.; Yoshihara, E.; Nakae, T.; Nishino, T. Role of protein F in maintaining structural integrity of the Pseudomonas aeruginosa outer membrane. J. Bacteriol. 1989, 171, 983–990.
- Woodruff, W.A.; Hancock, R.E. Pseudomonas aeruginosa outer membrane protein F: Structural role and relationship to the Escherichia coli OmpA protein. J. Bacteriol. 1989, 171, 3304–3309.
- Wang, Y. The function of OmpA in Escherichia coli. Biochem. Biophys. Res. Commun. 2002, 292, 396–401.
- Khan, N.A.; Shin, S.; Chung, J.W.; Kim, K.J.; Elliott, S.; Wang, Y.; Kim, K.S. Outer membrane protein A and cytotoxic necrotizing factor-1 use diverse signaling mechanisms for Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microb. Pathog. 2003, 35, 35–42.
- Prasadarao, N.V.; Wass, C.A.; Weiser, J.N.; Stins, M.F.; Huang, S.H.; Kim, K.S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect. Immun. 1996, 64, 146–153.
- Killiny, N.; Rashed, A.; Almeida, R.P. Disrupting the transmission of a vector-borne plant pathogen. Appl. Environ. Microbiol. 2012, 78, 638–643.
- Lampe, D.J.; Lauzon, C.R.; Miller, T. Development of symbiotic control of Pierce’s Disease. 2016. Available online: https://biopesticide.ucr.edu/abstracts/assets/Lampe_abstract.pdf (accessed on 12 April 2020).
- Miller, T. Symbiotic Control in agriculture and medicine. Comp. Biochem. Physiol. A Mol. Integr. Physiol. Comp. Biochem. Physiol. Pt A 2007, 146.
- Azizi, A.; Arora, A.; Markiv, A.; Lampe, D.J.; Miller, T.A.; Kang, A.S. Ribosome display of combinatorial antibody libraries derived from mice immunized with heat-killed Xylella fastidiosa and the selection of MopB-specific single-chain antibodies. Appl. Environ. Microbiol. 2012, 78, 2638–2647.
- Feil, H.; Feil, W.S.; Lindow, S.E. Contribution of Fimbrial and Afimbrial Adhesins of Xylella fastidiosa to Attachment to Surfaces and Virulence to Grape. Phytopathology 2007, 97, 318–324.
- De La Fuente, L.; Burr, T.J.; Hoch, H.C. Autoaggregation of Xylella fastidiosa cells is influenced by type I and type IV pili. Appl. Environ. Microbiol. 2008, 74, 5579–5582.
- Hoch, H.C. Continued assessment of Xylella fastidiosa fimbrial adhesins as important virulence factors in Pierce’s disease: Influence of xylem sap, p 87–91. In Proceedings of the 2010 Pierce’s Disease Research Symposium; Esser, T., Ed.; California Department of Food and Agriculture: Sacramento, CA, USA, 2010.
- Li, Y.; Hao, G.; Galvani, C.D.; Meng, Y.; De La Fuente, L.; Hoch, H.C.; Burr, T.J. Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation and cell-cell aggregation. Microbiology 2007, 153 Pt 3, 719–726.
- Markiv, A.; Anani, B.; Durvasula, R.V.; Kang, A.S. Module based antibody engineering: A novel synthetic REDantibody. J. Immunol. Methods 2011, 364, 40–49.
- Markiv, A.; Beatson, R.; Burchell, J.; Durvasula, R.V.; Kang, A.S. Expression of recombinant multi-coloured fluorescent antibodies in gor -/trxB- E. coli cytoplasm. BMC Biotechnol. 2011, 11, 117.
- Fang, W.; Vega-Rodriguez, J.; Ghosh, A.K.; Jacobs-Lorena, M.; Kang, A.; St Leger, R.J. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 2011, 331, 1074–1077.
- Bukhari, T.; Takken, W.; Koenraadt, C.J. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasites Vectors 2011, 4, 23.
- Miller, T.A. Paratransgenesis as a potential tool for pest control: Review of applied arthropod symbiosis. J. Appl. Entomol. 2011, 135, 474–478.
